Streptococcus pneumoniae antigen rapid kit
An in vitro diagnostic medical device that helps confirm pneumococcal infection by qualifying the Streptococcus pneumoniae antigen
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- South Korea
- Brand name
- LiliF® Streptococcus pneumoniae Ag Rapid kit
- Payment Terms
- T/T
- Production method
- Available
- Shipping / Lead Time
- Negotiable / Negotiable
- Keyword
- antigen, poct, rapid test kit, streptococcus pneumoniae
- Category
- Medical Test Kit

iNtRON Biotechnology, Inc.
Exhibition 1
Video-call Meeting Seongnam City 2022- Verified Certificate
-
17



| Product name | Streptococcus pneumoniae antigen rapid kit | Certification | - |
|---|---|---|---|
| Category | Medical Test Kit | Material | - |
| Keyword | antigen , poct , rapid test kit , streptococcus pneumoniae | Unit Size | - |
| Brand name | LiliF® Streptococcus pneumoniae Ag Rapid kit | Unit Weigh | - |
| origin | South Korea | Stock | 200 |
| Supply type | Available | HS code | 3822 |
Product Information
Pneumonia is not a big problem when it occurs in healthy people, but it is a very dangerous disease in children and the elderly. Therefore, it is important to diagnose the exact causative agent of pneumonia using an appropriate test method and to start effective antibiotic treatment at an early stage. The most common bacterial pneumonia causative agent in Korea is Streptococcus pneumoniae, accounting for approximately 30-40% of the total. Diagnosis of infection caused by Streptococcus pneumoniae is by traditional culture method using sputum or blood, but it must be diagnosed before antibiotic administration, and the reliability is low. However, immunochromatography using urine during antigen testing for Streptococcus pneumoniae has high sensitivity because it can detect C-polysaccharide in the cell wall for all species of Streptococcus pneumoniae.
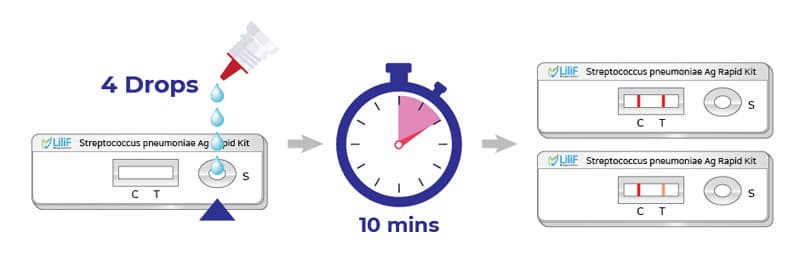
LiliF® Streptococcus pneumoniae Ag rapid kit uses ImmunoChromatographic Assay to diagnose pneumococcus, the causative agent of pneumonia, which is a major disease. can be read within 10 minutes).
• In vitro diagnostic medical device manufacturing license In vitro No. 22-80 - High-risk infectious agent gene test reagent
• An in vitro diagnostic medical device that helps confirm pneumococcal infection by qualifying the Streptococcus pneumoniae antigen (C-polysaccharide) in the urine sample of a patient with suspected pneumonia using ImmunoChromatographic Assay (ICA)
- Product Info Attached File
B2B Trade
| Price (FOB) | Negotiable | transportation | Air Transportation,Express |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | T/T | Shipping time | Negotiable |

- President
- Yoon Kyung-Won
- Address
- Seongnam-si, Jungwon-gu, Sangdaewon-dong, , 137 #605, Jungwon-gu, Seongnam-si, Gyeonggi-do, Korea
- Product Category
- Medical Test Kit,Other Extracts
- Year Established
- 1999
- No. of Total Employees
- 51-100
- Company introduction
-
iNtRON Bio has been focusing on the R&D investment since its foundation
and accelerates development speed after the IPO in KOSDAQ.
LiliF® is a specialized trademark of DR(Diagnosis) biz part of iNtRON Bio.
Also, we are developing diagnostic kits for various diseases.
We are aiming for the global R&D company and promoting the value of the company focused on
the Diagnostics, Resources and Molecular reagents. iNtRON’s production system is certified
by ISO9001:14000, ISO13485:GMP System and effectively supports product quality and manufacturing consistency.
■ Full line-up system in Diagnosis
Applicable in diverse markets from clinical diagnostics to basic science research Provides a total solution from screening with rapid test and to confirming via PCR
■ Customized Service
Less set up time / less errors / less contamination
■ Bulk & OEM Business
We can supply our products as your request
- Main Markets
-
 Bulgaria
Bulgaria
 Jordan
Jordan
 U.S.A
U.S.A
 Viet Nam
Viet Nam
- Main Product
Related Products

Urine Analysis Test Strip Self-Stik Series

R-ligo

PRO PRP KIT

Self-Stik Urine Strips
BioTracer Troponin I Rapid Test


































 South Korea
South Korea



