COVID-19 antigen rapid kit
Chromatographic immunoassay for the qualitative test for the detection of COVID-19 antigens in nasopharyngeal swab specimen of the suspected patients.
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- South Korea
- Brand name
- LiliF® GBN COVID-19 Ag Rapid Kit
- Payment Terms
- T/T
- Production method
- Available
- Shipping / Lead Time
- Negotiable / Negotiable
- Keyword
- antigen, rdt, covid-19 ag rapid, immunoassay diagnostics
- Category
- Medical Test Kit

iNtRON Biotechnology, Inc.
Exhibition 1
Video-call Meeting Seongnam City 2022- Verified Certificate
-
17



| Product name | COVID-19 antigen rapid kit | Certification | CE |
|---|---|---|---|
| Category | Medical Test Kit | Material | - |
| Keyword | antigen , rdt , covid-19 ag rapid , immunoassay diagnostics | Unit Size | - |
| Brand name | LiliF® GBN COVID-19 Ag Rapid Kit | Unit Weigh | - |
| origin | South Korea | Stock | 200 |
| Supply type | Available | HS code | 3822 |
Product Information
There are four genes in the Coronavirus family. Those are known to alpha, beta, gamma, and delta. Alpha and beta corona viruses can cause illness in both humans and animals, whereas others, such as gamma and delta coronaviruses, only infect animals. Reported illnesses have ranged from mild cold symptoms by Coronavirus 229E, NL63, OC43, or HKU1 to severe illness (e.g., pneumonia) by MERS-CoV and SARS-CoV. COVID-19 is a new coronavirus that has not previously identified. The new coronavirus
(COVID-19) belongs to beta and is one of the new infectious corona viruses that infects the human body as a pathogen of mass pneumonia that occurred in Wuhan, Hubei, China in December 2019. Common characteristics of people infected with coronavirus are fever, cough,shortness of breath and dyspnea. In more severe cases, it can cause
pneumonia, severe acute respiratory syndrome, kidney failure, and even death.
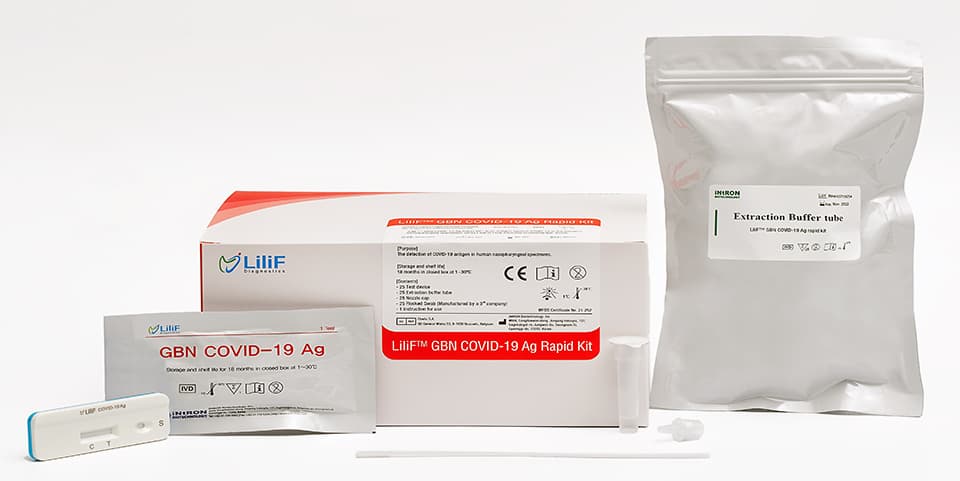
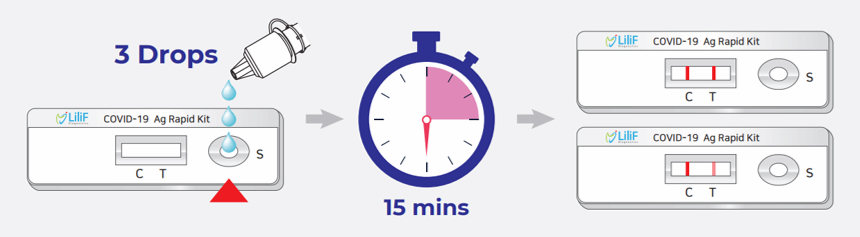
LiliF® GBN COVID-19 Ag Rapid Kit is a lateral flow chromatographic immunoassay for the detection of SARS-CoV-2 specific antigens in human nasopharyngeal swab specimens. It is intended to be used by professionals as an aid in the diagnosis of infection with SARS-CoV-2 coronavirus, which causes COVID-19 disease. After applying the extracted swab specimen onto the hole of testing device, human’s sample first react with gold conjugated mouse monoclonal antibody to SARS-CoV-2 Nucelcapsid antibody and rabbit anti-chicken IgY antibody. After that, each reactant diffuses on the membrane to the another monoclonal antibody to SARS-CoV-2 Nucelcapsid antibody marked as test lines and the control line is coated with chicken IgY antibody. If positive, the test line turns to red color because antigen-antibody-gold conjugate complex is formed on the test lines. The control line is intended for procedural control and should always appear if the test procedure is performed correctly and the control line reagents are operated.
LiliF® GBN COVID-19 Ag rapid kit is a chromatographic immunoassay for the qualitative test for the detection of COVID-19 antigens in nasopharyngeal swab specimen of the suspected patients. This kit should only be used as a diagnosis for screening purposes. In the case of positive results, confirmation tests through alternative diagnostic methods are required. After analyzing these test results and other clinical data, the Principal Investigator should perform a final diagnosis.
- Product Info Attached File
-
Brochure(RDT) - LiliF™ GBN COVID19 Ag Rapid kit - 21.06.07.pdf
- Verified Certificate
-

B2B Trade
| Price (FOB) | Negotiable | transportation | Air Transportation,Express |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | T/T | Shipping time | Negotiable |

- President
- Yoon Kyung-Won
- Address
- Seongnam-si, Jungwon-gu, Sangdaewon-dong, , 137 #605, Jungwon-gu, Seongnam-si, Gyeonggi-do, Korea
- Product Category
- Medical Test Kit,Other Extracts
- Year Established
- 1999
- No. of Total Employees
- 51-100
- Company introduction
-
iNtRON Bio has been focusing on the R&D investment since its foundation
and accelerates development speed after the IPO in KOSDAQ.
LiliF® is a specialized trademark of DR(Diagnosis) biz part of iNtRON Bio.
Also, we are developing diagnostic kits for various diseases.
We are aiming for the global R&D company and promoting the value of the company focused on
the Diagnostics, Resources and Molecular reagents. iNtRON’s production system is certified
by ISO9001:14000, ISO13485:GMP System and effectively supports product quality and manufacturing consistency.
■ Full line-up system in Diagnosis
Applicable in diverse markets from clinical diagnostics to basic science research Provides a total solution from screening with rapid test and to confirming via PCR
■ Customized Service
Less set up time / less errors / less contamination
■ Bulk & OEM Business
We can supply our products as your request
- Main Markets
-
 Bulgaria
Bulgaria
 Jordan
Jordan
 U.S.A
U.S.A
 Viet Nam
Viet Nam
- Main Product
Related Products

Nurugo CPR manikin

For women hygiene -Vaginal Irrigation Suction System : APRO-110

R-ligo
BioTracer Cardiac 3 in 1 Rapid Test

Covid 19 Detection


































 South Korea
South Korea



