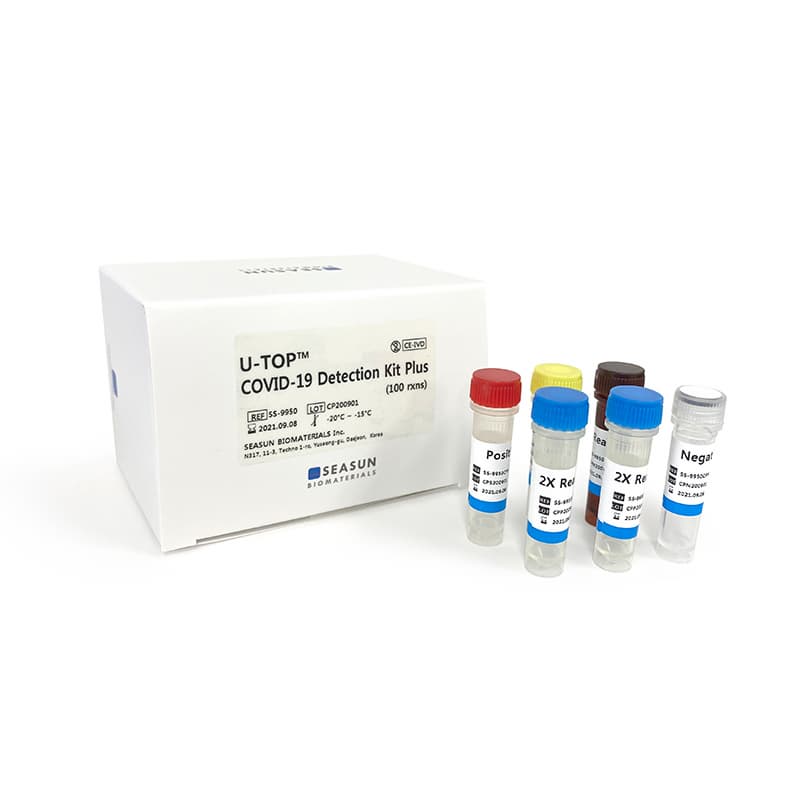
U-TOP COVID-19 Detection Kit Plus
COVID-19 TEST KIT (* 4-genes multi-detection for SARS-CoV-2)
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- South Korea
- Brand name
- COVID-19
- Payment Terms
- Others
- Production method
- Available
- Shipping / Lead Time
- Negotiable / Negotiable
- Category
- Medical Test Kit
SEASUN BIOMATERIALS Inc.
- Verified Certificate
-
3



| Product name | U-TOP COVID-19 Detection Kit Plus | Certification | FDA , CE |
|---|---|---|---|
| Category | Medical Test Kit | Material | - |
| Keyword | diagnostics kit , covid19 , precise dianosis , multi detection kit | Unit Size | 100.0 * 65.0 * 65.0 mm |
| Brand name | COVID-19 | Unit Weigh | 0 g |
| origin | South Korea | Stock | 0 |
| Supply type | Available | HS code | 3822001099 |
Product Information
U-TOP COVID-19 Detection Kit Plus (MFDS, CE-IVD)
U-TOP COVID-19 Detection Kit Plus is a real time PCR based in-vitro diagnostic medical device that is designed to detect presence of SARS-CoV-2 RNA through simultaneous examination of the SARSCoV-2 specific ORF1ab/RdRp, N, E and S gene using the nucleic acid extracted from the respiratory tract specimens from the individuals with signs and symptoms of infection who are suspected of COVID-19. The kit uses human RNase_P as an endogenous control for evaluation of RNA extraction efficiency and assay performance.
- Product Info Attached File
- Verified Certificate
-


B2B Trade
| Price (FOB) | Negotiable | transportation | Negotiation Other |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | Others | Shipping time | Negotiable |
- President
- Park Hee Kyung
- Address
- N317 11 3 Techno 1 ro, Yuseong-gu, Daejeon, Korea
- Product Category
- Medical Test Kit
- Year Established
- 2012
- Company introduction
-
- Main Product
Related Products

Covid 19 Detection

PREGABLE Ovulation Pregnancy Tests Kit with APP, SmileReader

R-ligo
BioTracer Cardiac 3 in 1 Rapid Test
BioTracer FOB Rapid Test


































 South Korea
South Korea


