Evaluation Kit for reflective SDPPG sensor
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- Brand name
- APMKr
- Payment Terms
- Negotiable
- Production method
- Negotiable
- Shipping / Lead Time
- Negotiable / Negotiable
- Keyword
- sdppg evauation kit
- Category
- Rehabilitation Therapy products

APMKorea
- Verified Certificate
-
17


| Product name | Evaluation Kit for reflective SDPPG sensor | Certification | - |
|---|---|---|---|
| Category | Rehabilitation Therapy products | Ingredients | - |
| Keyword | sdppg evauation kit | Unit Size | - |
| Brand name | APMKr | Unit Weigh | - |
| origin | Stock | - | |
| Supply type | - | HS code | - |
Product Information
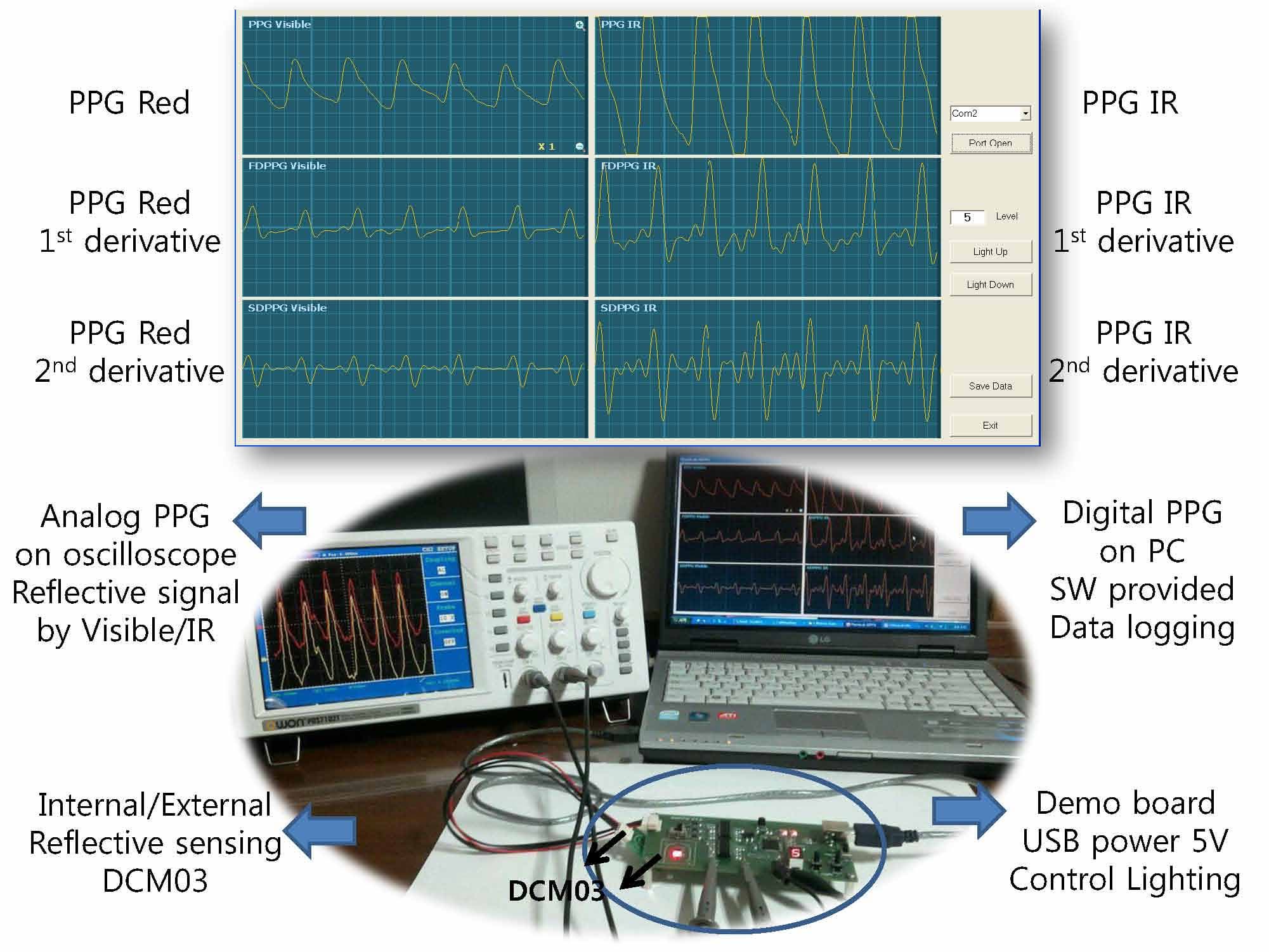
IR PPG, 1st derivative PPG, 2nd derivative PPG
USB power 5V, communicate PC
Control Lighting
Digital PPG on PC
SW provided, Data logging
Analog PPG on oscilloscope
Reflective signal by Visible/IR
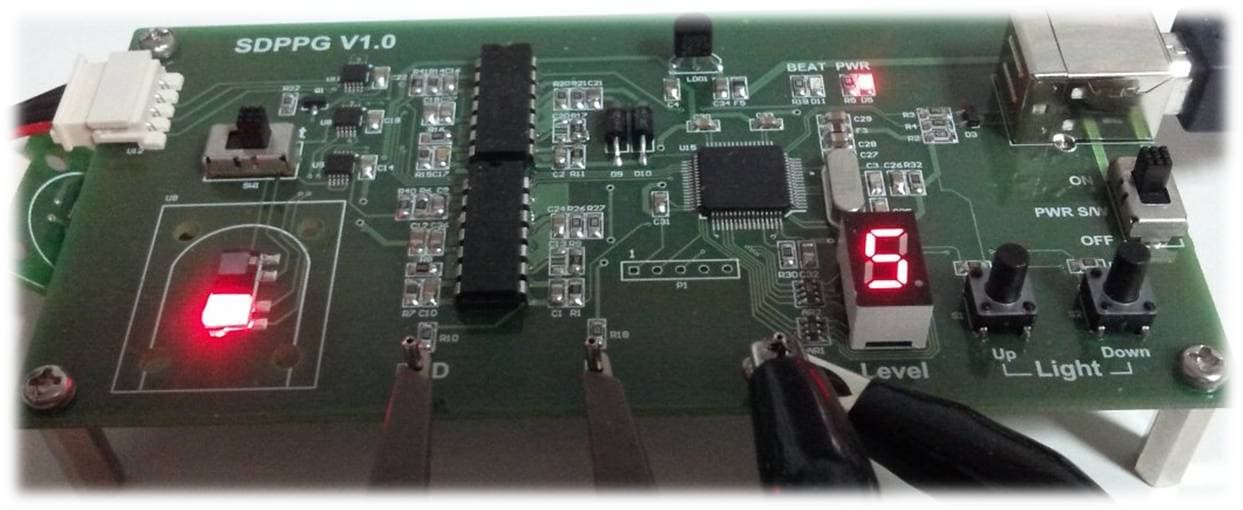
DCM03 Internal/External Reflective sensing
- Product Info Attached File
B2B Trade
| Price (FOB) | Negotiable | transportation | - |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | Negotiable | Shipping time | Negotiable |

- President
- shoon Han
- Address
- 52 Songnimro, Yuseong-gu, Daejeon, Korea
- Product Category
- Sensors
- Year Established
- 2008
- No. of Total Employees
- 1-50
- Company introduction
-
Shoon Han, CEO/owner has established APM (Advanced Photosensor Manufacturing) Korea on 9th, September 2008.
He had experience for 25years in semiconductor industry.
Company registrered Korea government as firm of manufacturer of electronics with certificate No. 305-27-21270 .
Joined the member of the KITA (Korea International Trade Association) Register No. 16118892.
Shipped SPO2 oximetry optic sensor for medical device customers in Germany, India, Indonesia, Singapore, Turkey and Korea. June 2009~.
Developed reflective PPG sensor, providing new idea and varial applications for medical device customers in Singapore, Germany, Ukraine, India. March 2010~.
Joined New Exporters 300 as a member, consulted by KITA (Korea International Trade Association). 23rd March 2010.
Shipped SPO2 sensor chips for medical device customers in Switzerland and Brazil May 2010~.
Joined Exporter Club as a member, consulted by SMBA (Small & Medium Business Administration). 18th May 2010.
Shipped PPG sensor for medical device customers in Colombia 20th May 2010~.
Presented at the Technology Road Show in Shanghai & Beijing China 6~10th Sep. 2010
Shipped Refective PPG sensor to Japan 15th Sep. 2010~.
Shipped SPO2 oximetry sensor for medical device customers in Shanghai China Dec 3rd 2010~.
Shipped PPG sensor chips for medical device customers in Uruguay 11th Dec 2010~.
Shipped Refective PPG sensor for medical device customers in South Africa 13rd Dec 2010~.
Assign a distributor for Shenzhen China medical market 13rd Dec 2010~.
Shipped SPO2 oximetry optic sensor for medical device to India, Israel, Vietnam, US, New zealand, UK, France, Slovenia, Bulgaria, Italy, Poland 2011~.
Transmitted SpO2 oximetery optic sensor Qualified by Honeywell in Germany, start to supply 2011~
Shipped first SPO2 blood sensor for medical device to India and Argentina Sep 2011~
Shipped SPO2 blood sensor and module for medical device to Philippine Oct 2011~
Had exhibitions, Hochiminh Vietnam 3~5th Nov, and Daejeon Korea 16~18th Nov 2011.
Shipped DCM02 repeat order by wearable application in US Nov 2011
I Interviewed by KITA, on the International Trade Jan. 2012
Developed DCM03 to maximize lighting current 2012.
Have exhibition, APM in 9th WTA Hitech 22~24th May 2012
Supplied APM sensors to 37 countries by Feb 2013
Have exhibition with Taiwan agent, APM in Medicare Taiwan 2013
Tested and work with Texas Instrument, AFE4490 with DCM03 Mesurement Results Aug 27, 2013
Developed smallest DCM05 4.8x2.6x1.0mm for mobile devices 31 Dec 2013.
TI designed DCM03+AFE4403+MSP430 module for the wearable SpO2 devices June 2014, DCM03 Miniaturized Pulse Oximeter Design by TI
Mocacare developped a wearable device with DCM03 Jan 2015, www.mocacare.com
A sister company APMSensor was established 19 Aug 2016, supporting marketing and sales for APMKorea.
Developed smallest DCM06 DCM07 DCM08 DCM10 DCM06B DCM08B in a row for mobile devices ~Nov 2017.
DCM06 DCM07 DCM06B was developped and using for wrist artery BP application of Chronisense Israel.
APMKorea and APMSensor have moved office to new place, 52 Songnim-ro Daejeon Korea Nov 2016.
Developed DCM07T which is merged to check the body temperature, registered a Patent(10-1982576).
APM in Medicall Chennai2019 India, Booth 3C3 Hall3 25~28th July.
APM_KOTRA (Korea Trade-Investment Promotion Agency) office in Chennai India 2019~.
Developed DCM11F merged to check the body temperature, pressure and ECG for a watch type blood pressure wearable device, registrated 2nd patent 2022.
Supplying SpO2 oximetry optic sensors DDL2002M & DDN2092M for "COVID19 Home Care" by Korean government (KDCA) 2021~2022.
Developed 4wavelengths DDL2008M and Largest area 5x5mm PD DDN2094for Mindray China March 2022.
Developed DCM12, 3arrays in SWIR (1300, 1600nm) + compound PD May 2024.
- Main Markets
-
 China
China
 Germany
Germany
 India
India
 Saudi Arabia
Saudi Arabia
 U.S.A
U.S.A
- Main Product
- Attached File


































 South Korea
South Korea