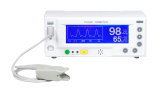
ACCURO
Versatile Bedside Pulse Oximeter
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- South Korea
- Payment Terms
- T/T
- Production method
- Available
- Shipping / Lead Time
- Negotiable / Negotiable
- Keyword
- medical device, pulse oximeters
- Category
- Oximeter
| Product name | ACCURO | Certification | - |
|---|---|---|---|
| Category | Oximeter | Material | - |
| Keyword | medical device , pulse oximeters | Unit Size | - |
| Brand name | - | Unit Weigh | - |
| origin | South Korea | Stock | 60 |
| Supply type | Available | HS code | - |
Product Information
- FDA Approved
- Nellcor Compatible probes
- Convenient built-in Power unit
- Perfusion rates from 0.05~20%
- 4 screen modes
- Electro surgical unit noise protected
- 15 days trend memory /10seconds
- Broader uses from neonates to the elderly
- 6hours charging time
- User friendly interface
- Audible and visible alarm
- IV pole mounting support (OPTION)
- Protective Carrying bag (OPTION)
- DIGI-VIEW™ PC monitoring S/W Available (OPTION)
B2B Trade
| Price (FOB) | Negotiable | transportation | Air Transportation |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | T/T | Shipping time | Negotiable |

- President
- Lee Dong Hwa
- Address
- Gasan-dong, Korea Techon B/D. 715,#709, Geumcheon-gu, Seoul, Korea
- Product Category
- Other Health Care Products
- Year Established
- 2004
- Company introduction
-
Charmcare developed the world's first truly wearable blood pressure monitor
- Main Markets
-
 Bangladesh
Bangladesh
 Egypt
Egypt
 Poland
Poland
 Turkey
Turkey
 U.S.A
U.S.A
- Main Product



































 South Korea
South Korea