Kbelle ULTRA , Hyaluronic Acid Dermal Filler, HA Filler, Facial Filler, Korean Filler, Face Care
Sterile Absorbable Hyaluronic Acid Dermal Filler, Patented MCL Technological Long Lasting HA Filler
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- South Korea
- Brand name
- Kbelle
- Payment Terms
- Others,T/T
- Production method
- ODM
- Shipping / Lead Time
- Negotiable / Negotiable
- Keyword
- anti aging, dermal filler, ha filler, face treatment

ABLife Global Co., Ltd.
- Verified Certificate
-
2

| Product name | Kbelle ULTRA , Hyaluronic Acid Dermal Filler, HA Filler, Facial Filler, Korean Filler, Face Care | Certification | - |
|---|---|---|---|
| Category |
Other Beauty Products
Other Beauty Appliance Other Facial Care Other Medical Consumables |
Ingredients | HYALURONIC ACID |
| Keyword | anti aging , dermal filler , ha filler , face treatment | Unit Size | 180.0 * 80.0 * 30.0 mm |
| Brand name | Kbelle | Unit Weigh | - |
| origin | South Korea | Stock | 1000 |
| Supply type | ODM | HS code | - |
Product Information
Kbelle-Hyaluronic Acid Fillers
The skin's HA levels decline year after year, skin becomes thinner, drier and loses its elasticity. Lines and wrinkles start to appear and the entire harmony of the face changes. So Hyaluronic acid (HA) is essential for maintaining moisture, firmness and elasticity of the skin.
Innovative Technology
Multi Staged Cross Linking(MCL)
High viscosity is obtained through patented stage micro beads manufacturing technology based on cross linked Hyaluronic Acid.
We adjusted cross-linking rate according to product characteristics. We produced a various of high quality K Belle depending on area.
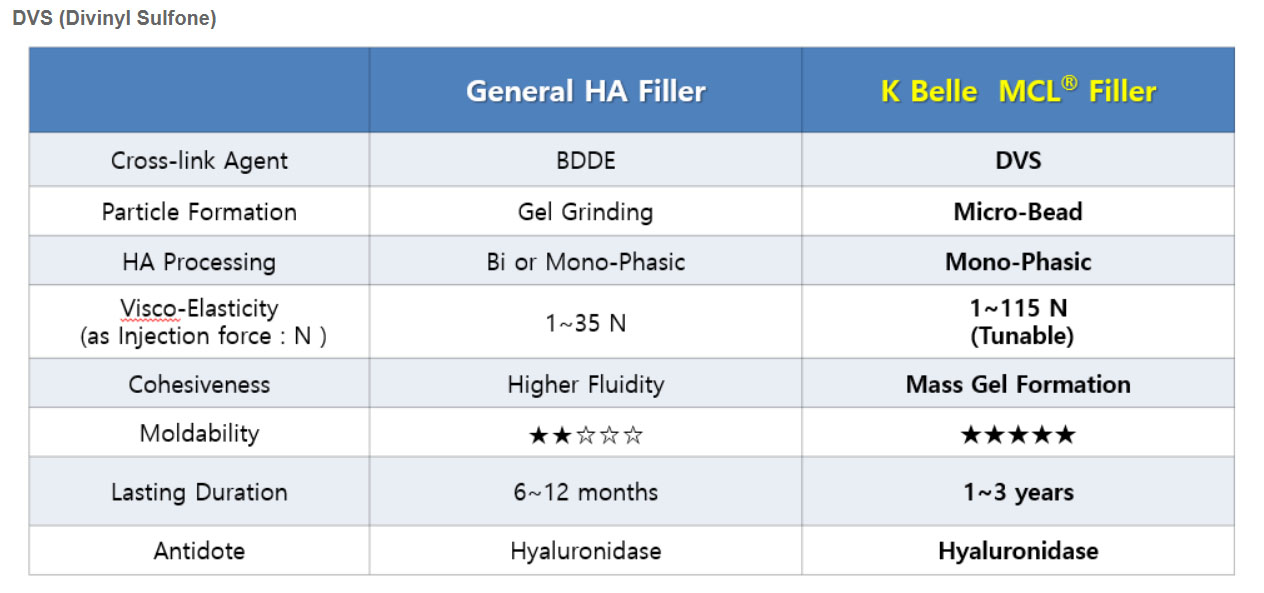
Strong Points of DVS Filler
Long-Lasting Mechanism (High cohesiveness and viscosity)
Strong Gel Hardness
Easy Molding
Easily Removable(safe)
Cross-linked HA
K Belle has developed MDAWO technology (Multiple Degree Amphiphilic Wash-Out technology) and applied it to processes.
The process of technology, the DVS component is completely removed and none of un-cross linked DVS is detected.
In general, the crosslinking process is carried out using DVS having a purity of about 95% for BDDE used for crosslinking
HA and 97% or more purity for EXTRA. By using highly pure crosslinking bridge, crosslinking ratio is high, and high
quality products can be produced.
K Belle : Quality Assurance
1. Safe Material
Shiseido Co., LTD. in Japan with European Pharmacopeia(EP) Grade
2. Strict Review Process
Developed DVS, MDAWO technology (Multiple Degree Amphiphilic Wash-Out technology) with purity of 97% or more and applied process.
3. Stability and Effectiveness
KFDA for medical device on 4TH grade and CE, and DVS Washing technology.
K Belle can be used for specific areas, ensures a safe and effective result.
Face : Areas Used
K Belle Soft
Fine wrinkles on eye rim and sides of mouth
Improvement of skin elasticity
K Belle Mid
Nasolabial, Frown Lines, Lips
K Belle Ultra
Cheekbones, Nose, Chin
Body : Areas used
K Belle S
1. Increase fullness and projection of our breasts.
2. Improve the balance of our figure.
K Belle H
1. Increase buttocks size.
2. Lift saggy bottom.

B2B Trade
| Price (FOB) | Negotiable | transportation | Air Transportation,Negotiation Other |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | Others,T/T | Shipping time | Negotiable |

- President
- YONG DAE MOON
- Address
- Saemal-ro, 102,#819, Guro-gu, Seoul, Korea
- Product Category
- Face Mask,Medical Face Mask,Other Beauty Products,Other Facial Care,Other Medical Consumables
- Year Established
- 2022
- No. of Total Employees
- 1-50
- Company introduction
-
- Main Markets
-
 Brazil
Brazil
 France
France
 Japan
Japan
 U. Kingdom
U. Kingdom
 U.S.A
U.S.A
- Main Product







































 South Korea
South Korea

_2.jpg)
_2.jpg)

_2.jpg)
