Health Mask
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- South Korea
- Brand name
- MK SHARON
- Payment Terms
- L/C,T/T
- Production method
- Negotiable
- Shipping / Lead Time
- Negotiable / Negotiable
- Keyword
- face mask, mask, health mask, covid-19
- Category
- Medical Face Mask
MK Medical Device
- Country / Year Established
-
 South Korea
/
2016
South Korea
/
2016
- Business type
- Manufacturer
- Verified Certificate
-
5


| Product name | Health Mask | Certification | - |
|---|---|---|---|
| Category | Medical Face Mask | Ingredients | - |
| Keyword | face mask , mask , health mask , covid-19 | Unit Size | - |
| Brand name | MK SHARON | Unit Weigh | - |
| origin | South Korea | Stock | - |
| Supply type | - | HS code | 630790 |
Product Information
We are a specialized manufacturer for Face/Health Masks which can overcome COVID-19 crisis.
We have two running qualities for Face/Health Mask made by us, all of which were registered to USFDA as follows.
* USFDA Manufacturer Owner/Operator # : 10077093
* USFDA Device Listing # : D415213
(1) KF-94 :
(a) Product : Equivalent quality to N95(USA) and FFP2(EU), showing at least 94% filtration efficiency against the particles in size 0.4um.
(b) Package : 1pc/pouch
(2) KF-AD :
(a) Anti-droplets mask, showing excellent waterproof effect and still much helpful against COVID-19 accordingly.
(b) Package : 5pcs/pouch
Packaging/packing can be adjusted as per buyer's request.
The shape for both is 3D design, which is easy to breathe.
B2B Trade
| Price (FOB) | Negotiable | transportation | - |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | L/C,T/T | Shipping time | Negotiable |
- President
- KANG JE WON
- Address
- 54 Eungyejungangro 306beongil, Siheung-si, Gyeonggi-do, Korea
- Product Category
- Medical Face Mask
- Year Established
- 2016
- No. of Total Employees
- 1-50
- Company introduction
-
We are a specialized manufacturer for Face/Health Masks which can overcome COVID-19 crisis.
We have two running qualities for Face/Health Mask made by us, all of which were registered to USFDA as follows.
* USFDA Manufacturer Owner/Operator # : 10077093
* USFDA Device Listing # : D415213
(1) KF-94 :
(a) Product : Equivalent quality to N95(USA)/FFP2(EU)/KN95(China), showing at least 94% filtration efficiency against the particles in size 0.4um.
(b) Package : 1pc/pouch
(2) KF-AD :
(a) Product : Equivalent quality to FFP1(EU)/KN90(China), showing at least 90% filtration efficiency against the particles in size 0.6um.
(b) Package : 5pcs/pouch
The shape for both is 3D design, comfortable to wear and breathe.
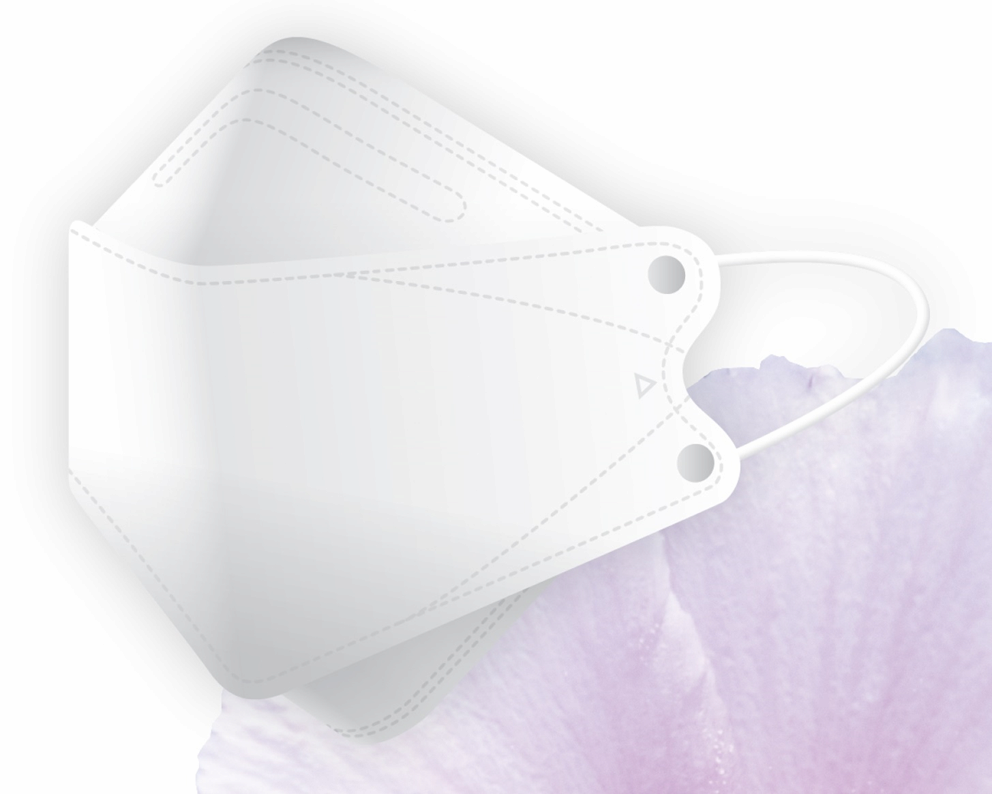
- Main Product
Related Products
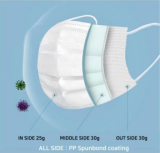
Disposal Mask 3 layer, AD, KF94 KF80

Soomplus XG

4-Ply Disposable KF94 Sanrio Hello Kitty My Melody Character Print Kids Child Face Masks Korea

NEW 3-Ply Reusable Washable Breathable Fabric Premium Face Mask for Adult, Teens Made in Korea
Surgical / Medical Mask, shoe cover, hair cap







































