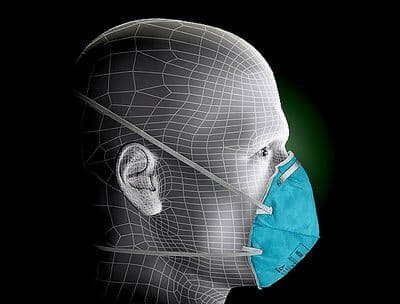
Surgical Mask 1860, N95
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- Austria
- Payment Terms
- L/C,T/T
- Production method
- Negotiable
- Shipping / Lead Time
- Negotiable / Negotiable
- Keyword
- n95 mask, surgical mask, n95 face mask, 3m 1860
- Category
- Medical Face Mask
7STARS CO.,Ltd.
- Country / Year Established
-
 South Korea
/
2015
South Korea
/
2015
- Business type
- Manufacturer
- Verified Certificate
-
4


| Product name | Surgical Mask 1860, N95 | Certification | - |
|---|---|---|---|
| Category | Medical Face Mask | Ingredients | - |
| Keyword | n95 mask , surgical mask , n95 face mask , 3m 1860 | Unit Size | - |
| Brand name | - | Unit Weigh | - |
| origin | Austria | Stock | - |
| Supply type | - | HS code | 630790 |
Product Information
3M Surgical Mask 1860, N95
Details
NIOSH Approved: N95
FDA Cleared for use as surgical mask
Helps protect against certain airborne biological particles
Fluid resistant and disposable
SKU: 70070612364 UPC: 50707387419429
This health care particulate respirator and surgical mask helps provide respiratory protection against certain airborne biological particles. It is disposable and fluid resistant to splash and spatter of blood and other infectious material.
This healthcare respirator is designed to help provide respiratory protection for the wearer. It meets CDC guidelines for Mycobacterium tuberculosis exposure control. As a disposable particulate respirator, it is intended to help reduce wearer exposure to certain airborne particles including those generated by electrocautery, laser surgery, and other powered medical instruments. As a surgical mask, it is designed to be fluid resistant to splash and spatter of blood and other infectious materials.
Features include:
NIOSH approved N95
Meets CDC guidelines for Mycobacterium tuberculosis exposure control
FDA cleared for use as a surgical mask
99% BFE (Bacterial Filtration Efficiency) according to ASTM F2101
Fluid resistant according to ASTM F1862
Respirator contains no components made from natural rubber latex
Collapse resistant cup shape design
Braided headbands, cushioning nose foam, and light weight construction for comfortable wear
Suggested settings and applications: Operating Rooms, Clinics, TB Wards, Patient Care, Labor and Delivery, Infection Control Practices, Laboratory, emergency or pandemic preparedness planning, stockpiling, etc.
WARNING: These respirators help reduce exposures to certain airborne contaminants. Before use, the wearer must read and understand the User Instructions provided as a part of the product packaging. In the U.S., a written respiratory protection program must be implemented meeting all the requirements of OSHA 1910.134 including training, fit testing and medical evaluation. In Canada, CSA standards Z94.4 requirements must be met and/or requirements of the applicable jurisdiction, as appropriate. Misuse may result in sickness or death. For proper use, see package instructions.
Made in EU
B2B Trade
| Price (FOB) | Negotiable | transportation | - |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | L/C,T/T | Shipping time | Negotiable |
- President
- EOM GI SAN
- Address
- 193 Deogi-ro Ilsanseo-gu Goyang-si Gyeonggi, Ilsanseo-gu, Goyang-si, Gyeonggi-do, Korea
- Product Category
- Medical Face Mask,Other Medical Consumables
- Year Established
- 2015
- No. of Total Employees
- 1-50
- Company introduction
-
As a generaltrading company, our company has been global trading by making eco-friendly
raw materials andindependent products for 6 years.
Now, we areactively producing products related to covid-19. The N95, KF94, 3M 1860 masks made in Korea (KFDA-certified), covid diagnostic kits based on nucleic-acids and bodybags made from eco-friendly materials are KC-certified and RoHS certification.As such, the company is actively engaged in business, focusing on variousmedical supplies and products related to personal hygiene.
- Main Product
Related Products

Cleanroom Mask
__2.jpg)
Disposable Health Mask (KF94)

4-Ply Disposable KF94 Sanrio Hello Kitty My Melody Character Print Kids Child Face Masks Korea

Brand New 4Ply KF94 Disposable Nintendo Pikachu Squirtle Print Kids Child Face Mask Made in Korea
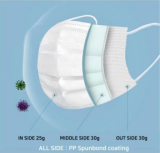
Disposal Mask 3 layer, AD, KF94 KF80