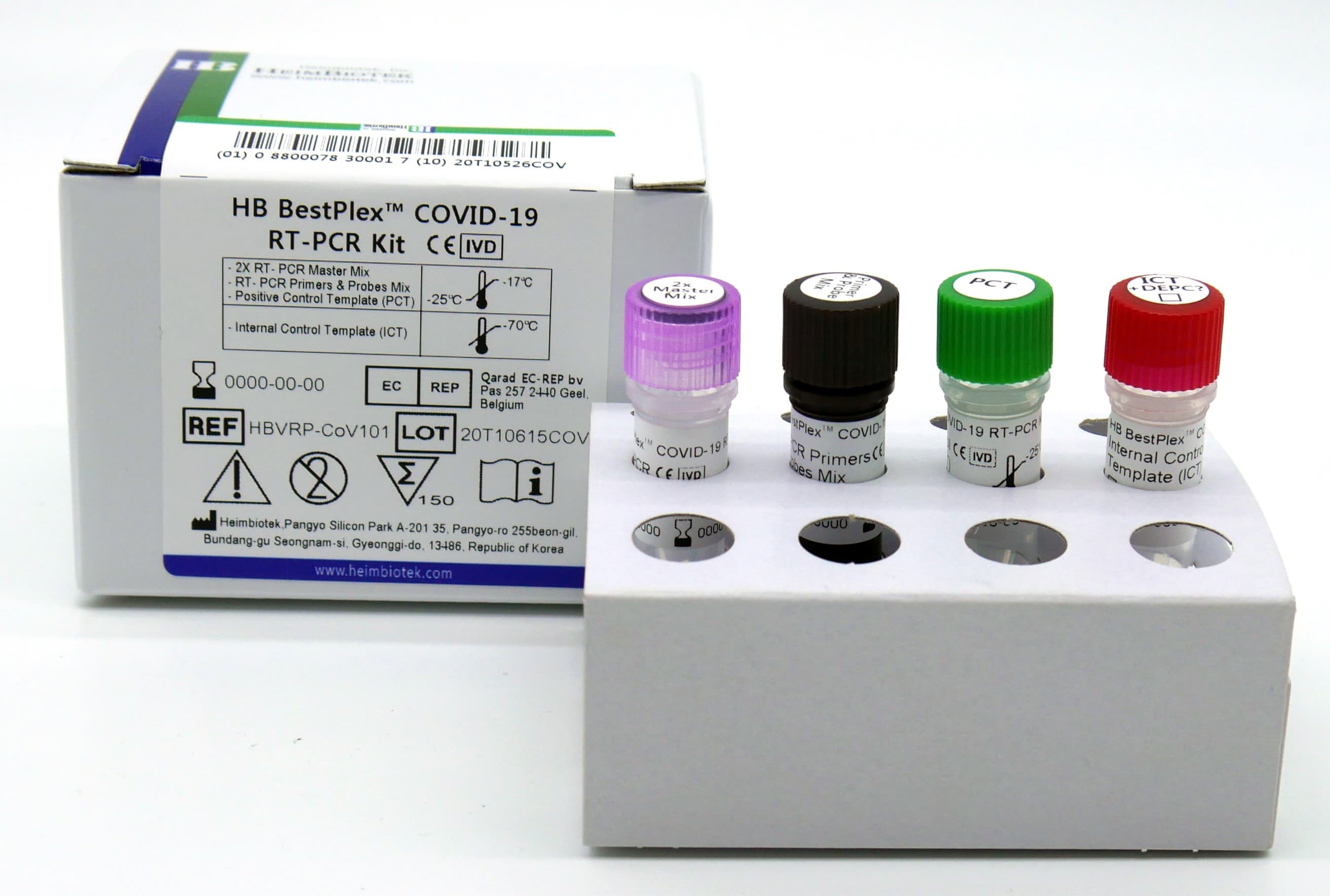
HB BestPlex™ COVID-19 RT-PCR Kit
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- South Korea
- Brand name
- HB BestPlex™
- Payment Terms
- T/T
- Production method
- Negotiable
- Shipping / Lead Time
- Negotiable / Negotiable
- Keyword
- diagnostics kit, pcr test kit, virus, covid19
- Category
- Medical Test Kit

HeimBiotek
- Verified Certificate
-
9


| Product name | HB BestPlex™ COVID-19 RT-PCR Kit | Certification | CE |
|---|---|---|---|
| Category | Medical Test Kit | Ingredients | - |
| Keyword | diagnostics kit , pcr test kit , virus , covid19 | Unit Size | 78.0 * 55.0 * 64.0 mm |
| Brand name | HB BestPlex™ | Unit Weigh | 500 g |
| origin | South Korea | Stock | - |
| Supply type | - | HS code | 3822002019 |
Product Information
INTENDED USE:
The HB BestPlexTM COVID-19 RT-PCR Kit is a multiplex real-time RT-PCR test intended for the presumptive qualitative detection of nucleic acid from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in nasopharyngeal or oropharyngeal swab from individuals exhibiting signs and symptoms for COVID-19. Results of this RT-PCR test are for the identification of SARS-CoV-2 RNA. A positive result indicates the presence of SARS-CoV-2 RNA in the collected specimen. However, this does not rule out the possibility of co-infection with other viruses or bacteria. All positive results need to be reported to proper public health authorities in the United States. Negative results by this kit do not preclude SARS-CoV-2 infection. Definitive diagnosis must be considered with other clinical observations, patient history and epidemiological information. The HB BestPlexTM COVID-19 RT-PCR Kit is intended to use by well-trained laboratory personnel for the techniques and diagnostic procedure of real-time PCR. Testing with the HB BestPlexTM COVID-19 RT-PCR Kit is limited to laboratories - certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. §263a, to perform high complexity tests or by similarly qualified non U.S. laboratories.
The HB BestPlexTM COVID-19 RT-PCR Kit is a multiplex real-time RT-PCR test intended for the presumptive qualitative detection of nucleic acid from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in nasopharyngeal or oropharyngeal swab from individuals exhibiting signs and symptoms for COVID-19.
Results of this RT-PCR test are for the identification of SARS-CoV-2 RNA. A positive result indicates the presence of SARS-CoV-2 RNA in the collected specimen. However, this does not rule out the possibility of co-infection with other viruses or bacteria. All positive results need to be reported to proper public health authorities in the United States. Negative results by this kit do not preclude SARS-CoV-2 infection. Definitive diagnosis must be considered with other clinical observations, patient history and epidemiological information.
The HB BestPlexTM COVID-19 RT-PCR Kit is intended to use by well-trained laboratory personnel for the techniques and diagnostic procedure of real-time PCR. Testing with the HB BestPlexTM COVID-19 RT-PCR Kit is limited to laboratories - certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. §263a, to perform high complexity tests or by similarly qualified non U.S. laboratories.
SPECIFICATION:
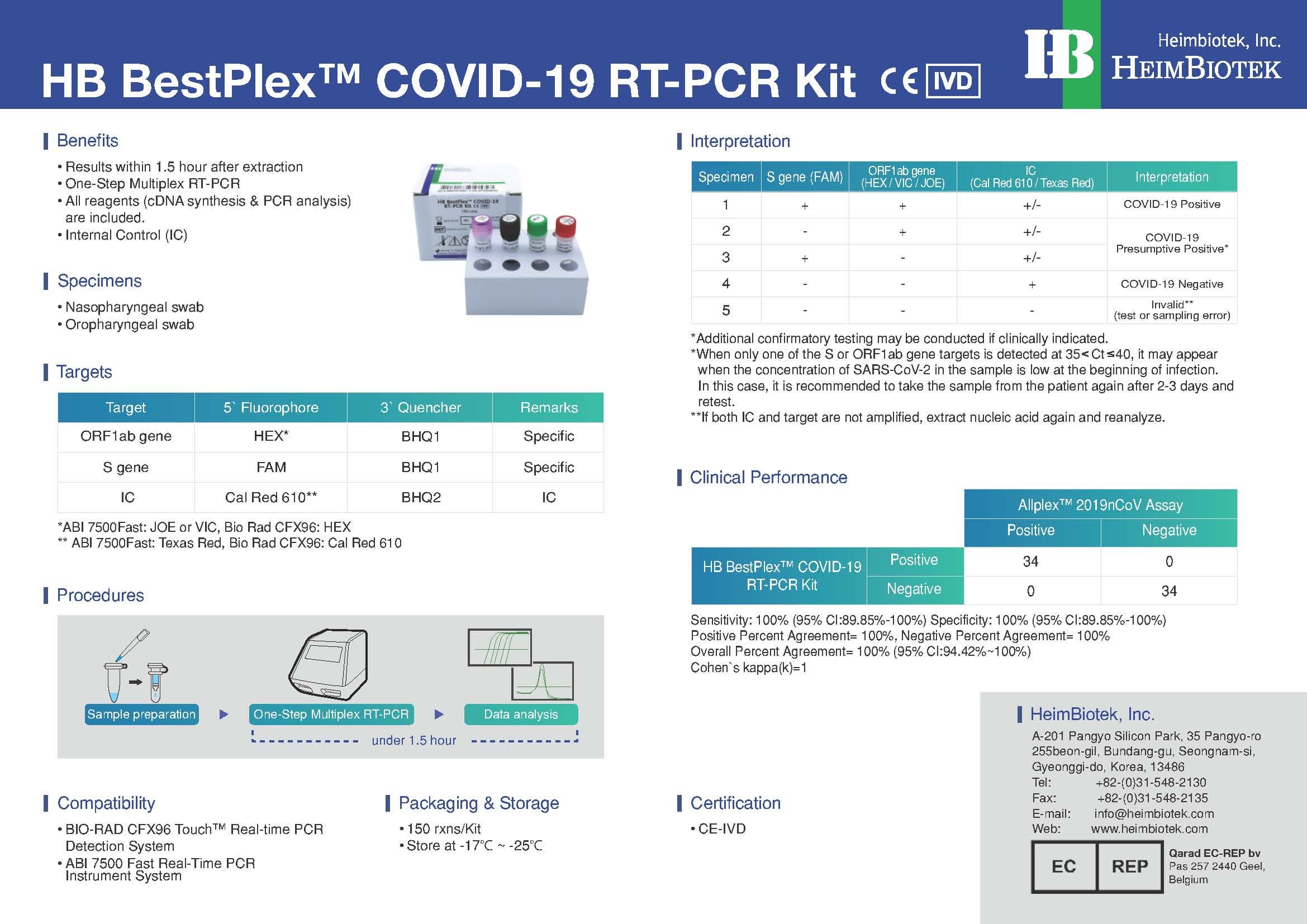
- Results within 1.5 hour after extraction
- One-Step RT Real-Time PCR (Only require RNA template)
- All reagents (cDNA synthesis & qPCR analysis) are included
- The validity of RT-PCR reaction is precisely verified by using Positive Control
COMPATIBILITY:
- CFX96 Real-time PCR Detection System-IVD [Bio-Rad]
- Applied Biosystems 7500 Fast Dx platform-IVD [ABI]
PACKAGE:
- 150 rxns/Kit [HBVRP-CoV101]
- 450 rxns/Kit [HBVRP-CoV201]
- 3,600 rxns/Kit [HBVRP-CoV301]
- Product Info Attached File
-
Clinical Trial Report_HeimBiotek_HB BestPlex COVID-19 RT-PCR Kit_20200513.pdf
HB-IFU-COV-01-EN-R0_Instruction for Use_20200616.pdf
HB COVID-19 RT-PCR Kit(Brochure)_20200618.pdf
HB BestPlexTM COVID-19 RT-PCR Kit Presentation Ver.2_20200714.pdf
- Verified Certificate
-

B2B Trade
| Price (FOB) | Negotiable | transportation | - |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | T/T | Shipping time | Negotiable |

- President
- Jae Hoon Lee
- Address
- Pangyo Silicon Park A-201 35, Pangyo-ro 255Beon-gil, Bundang-Gu Seongnam-Si, Gyeonggi-Do, KOREA
- Product Category
- Chemical Reagents & Products,Education Supplies,Other Chemical Machinery Equipment,Other Education Supplies
- Year Established
- 2014
- No. of Total Employees
- 1-50
- Company introduction
-
HeimBiotec,Ink. has been devoted to the global leader in genetic analysis and molecular diagnostics and is a R & D enterprise based on original technology secured through efficient and strategic research and development.
We are developig and researching Companion Diagnostics based on SBDEⓇ(Specific Bi-Direction Extension Technique. And we have released micro RNA detection assay kit which is based on the Reverse Tranx-x-scription quantitative PCR and It is higher than other products in the worldas the third commercializtion
we will continue to grow steadily through continuous R & D investment and new product development, We will achieve globalization of technology and product as commercializing genetic anaylsis and molecular diagnostic product with high technology and competitiveness.
- Main Markets
-
 Canada
Canada
 China
China
 Germany
Germany
 Japan
Japan
 Singapore
Singapore
 Taiwan
Taiwan
 Thailand
Thailand
 U.S.A
U.S.A
- Factory Information
-
Research Institute
- Main Product
Related Products
Covid 19 Detection

A+CheQ COVID-19 High-Speed RT-qPCR Detection Kit
AFP/PSA/CEA Rapid Test

R-ligo
COVID 19 IgM / IgG RAPID KIT


































 South Korea
South Korea