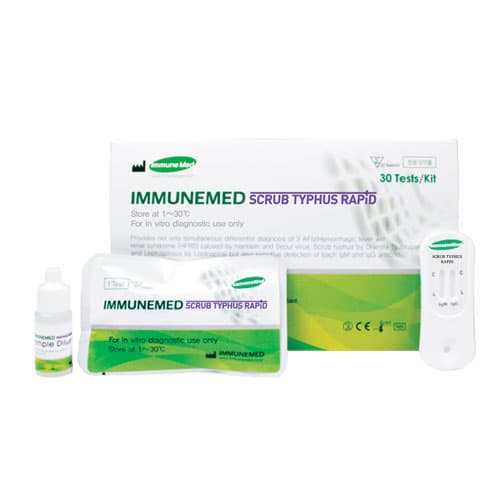
Scrub Typhus Rapid Kit
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- South Korea
- Payment Terms
- Negotiable
- Production method
- Negotiable
- Shipping / Lead Time
- Negotiable / Negotiable
- Keyword
- kit, diagnostic, scrub typhus
- Category
- Other Health Care Products
ImmuneMed
- Country / Year Established
-
 South Korea
/
2000
South Korea
/
2000
- Business type
- Manufacturer
- Verified Certificate
-
11


| Product name | Scrub Typhus Rapid Kit | Certification | - |
|---|---|---|---|
| Category | Other Health Care Products | Ingredients | - |
| Keyword | kit , diagnostic , scrub typhus | Unit Size | - |
| Brand name | - | Unit Weigh | - |
| origin | South Korea | Stock | - |
| Supply type | - | HS code | - |
Product Information
Scrub Typhus Rapid Kit
│ Descriptions │
"ImmnuneMed Scrub Typhus Rapid" tests the antibodies against Orientia tsutsugamushi in the patient's plasma, serum or blood using Lateral Flow ImmunoChromatographic Assay. After applying diluted serum, plasma or blood onto the hole of testing device, for IgM, patient`s sample first react with gold conjugated anti-human IgM antibody and for IgG, with gold conjugated protein A. After that, each reactant diffuses on the membrane to the recombinant antigens marked as test lines. If positive, the test line turns to red color because antigen-antibody-gold conjugate complex is formed on the test lines. AFI(Acute Febrile Illness) is urgenly required accurate differential early diagnosis and proper therapy against different causative microorganisms with similar clinical symptoms. This Scrub Typhus Rapid provides not only rapid diagnosis of Scrub Typhus (Tsutsugamushi disease) among AFI but also sensitive detection of each IgM and IgG antibody simultaneously.
│ Feature │
Testing device: one round hole for sample applying on the plastic cassette and two detection window for IgM and IgG. There are 2 different markings, such as C for control line and T for Scrub typhus (Tsutsugamushi disease) by Orientia tsutsugamushi on each detection window.
2) Sample diluent: colorless liquid in the semi-transparent plastic bottle
│ Specification │
(1) Use serum, plasma or whole blood as specimen.
(2) Collected blood containing anti-coagulant should be used immediately
or the blood kept in 2~8 ¡É for 3 days is possible to use.
(3) Separated serum and plasma could be used until one month if kept
in 2~8 ¡É or 1 year if kept frozen.
2) Methods
(1) If each specimen and reagent is kept frozen, place it for 30 min at room temperature before use.
(2) After pulling out the testing cassette from the closed pouch, place it on the flat position.
(3) In case of serum or plasma, 3 §¡ is diluted about 100 fold with 300 §¡ sample diluent. If whole blood, take 6 §¡ and it is diluted about 50 fold with 300 §¡ sample diluent. After that, apply 300 §¡ of this diluted sample to the hole. * Or when 3 §¡ of serum or plasma, or 6 §¡ of whole blood are directly applied to the sample pad in the hole, immediately add 7 drops of sample diluent provided.
(4) Wait for 15 min until the red line appears on the control line. Read and interpret the result exactly after 15 min from applying the diluted sample. Ignore the red line appeared after 15 min for interpretation.
3) Interpretation ¨ç Positive: Red line must appear on each C line of IgM and IgG in the detection windows and one or two red lines should appear on the test line, T in the windows (Fig 1. a,b,c).
¨è Negative: Red lines only appear on both control lines of IgM and IgG in the detection windows and no red line should appear on the test line, T (Fig 1. d).
¨é Invalid: If red line does not appear from at least one out of 2 C lines, this result is interpreted as invalid, and do test again (Fig 1. e).
4) Quality control
① Red lines should appear on both control lines for each testing. ② If the red line does not appear from at least one out of two C lines, re-test is recommended because the directions may not have been followed correctly or the testing device may have deteriorated.
B2B Trade
| Price (FOB) | Negotiable | transportation | - |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | Negotiable | Shipping time | Negotiable |
- President
- Kim Sun Mi
- Address
- Room #2-2 Bld.#3 Chuncheon BioTown, 32 Soyanggangro, Chuncheon, Gangwon-do, Republic of korea,200-957
- Product Category
- Examination & Testing Instrument
- Year Established
- 2000
- No. of Total Employees
- 1-50
- Company introduction
-
People are free from the threat and burden of diseases by medical technologies. ImmuneMed introduces a diagnostic kit for acute illnesses that will save numerous lives.
ImmuneMed Inc. is a company that specializes in developing new anti-viral drugs and in-vitro diagnostics. ImmuneMed’s patented ‘’ImmuneMed Leptospira Rapid Kit’’ is designed to detect leptospirosis quickly and accurately in its early stages using antibodies in the patient’s serum. The microscopic agglutination test, MAT, a gold standard method for diagnosing leptospirosis caused by over 200 different serotypes, has the downside of taking over two hours to reach a diagnosis.
However, ImmuneMed’s diagnostic kit uses a distinguished technology that detects leptospirosis by using genus specific antigen, which is created by optimizing to react with patient’s antibodies well.
Unlike MAT, it offers visible identification within 15 minutes. Furthermore, it has been proven through national and international clinical evaluation to have an over 90% on sensitivity and specificity.
The pre-test line, located below the test line, removes the possibility of misinterpreting the test results, leading to higher accuracy.
To use ImmuneMed’s diagnostic kit, first drop clinical sample onto the diagnostic kit. As the sample reaches the gold pad area, it is combined with the gold conjugated antibodies on the pad.
As the leptospira specific antibody in a sample continues to move toward the test line, it creates purple lines to indicate leptospirosis positive. Also, the remaining gold conjugated antibodies of the gold pad that didn’t react to the antibodies within the sample create a purple band at the contorl line to indicate the conformity of the test.
ImmuneMed’s in vitro diagnostic kit was developed through years of continuous R&D, and is capturing the global spotlight as a patented technology for leading mankind’s healthier life.
- Main Markets
-
 Laos
Laos
 Malaysia
Malaysia
 Philippines
Philippines
 Thailand
Thailand
- Main Product






































_2.jpg)

