Multiplex Human Renal Cell Carcinoma Detection Kit
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- Payment Terms
- Negotiable
- Production method
- Negotiable
- Shipping / Lead Time
- Negotiable / Negotiable
- Keyword
- Category
- Medical Test Kit
Genomine Inc.
- Country / Year Established
-
 South Korea
/
South Korea
/
- Business type
- Others
- Verified Certificate
-
13


| Product name | Multiplex Human Renal Cell Carcinoma Detection Kit | Certification | - |
|---|---|---|---|
| Category | Medical Test Kit | Ingredients | - |
| Keyword | - | Unit Size | - |
| Brand name | - | Unit Weigh | - |
| origin | Stock | - | |
| Supply type | - | HS code | - |
Product Information
Description
- Multiplex Human Renal Cell Carcinoma Biomarker Panel
Trade Name
- GenoPlex MAP
Background Information
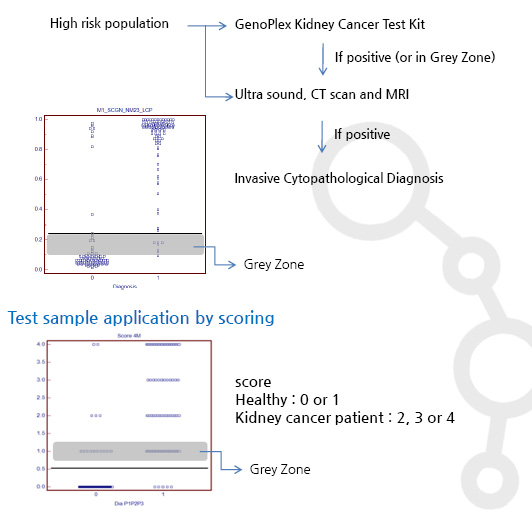
- GENOMINE Inc. offers the GENOPLEX MAP Human Renal Cell Carcinoma detection kit. The Cancer Biomarker Panel includes Four-Plex assay, which simultaneously measures three serum biomarkers, N-nicotinamide metyltransferase(NNMT), Non-metastatic cells 1(NM23) and L-plastin(LCP). The combination of the four serum biomarkers that discriminate between disease-free and Renal Cell Carcinoma patients was characterized and validated by GENOMINE Inc. and Yonsei University Medical School. Using a scoring algorithm, the profile of three biomarkers in circulation was used to detect all stages of Renal Cell Carcinoma patients with high sensitivity and accuracy. The assays can be used to quantify these biomarkers in serum, plasma, and cell/tissue culture supernatant samples.
Specification
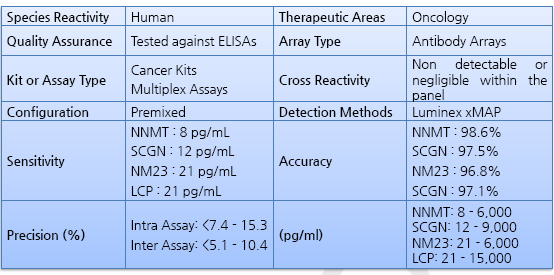
Advantages and Benefits
- Detecting of 3 kidney cancer markers in a single well
- Highly sensitive
- Multiplexing reduces costs and time
- Generates more data with less sample
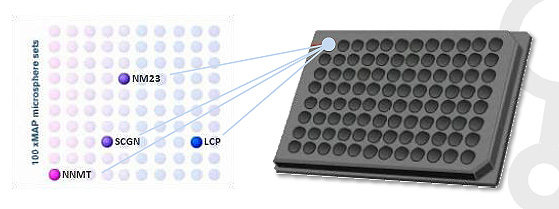
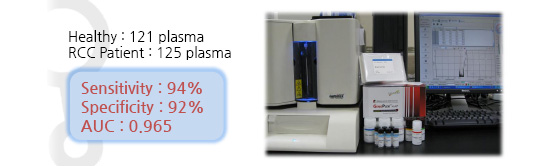
Clinical Test Results
- Assay : GenoPlex MAP (multiplex human renal cell carcinoma(RCC) biomarker test kit)
- Multiple marker data analysis : logistic regression & ROC
Application
- Screening test for kidney cancer in a high-risk population
- Screening test for Non- high risk population
- Treatment monitoring for post-surgery patients
B2B Trade
| Price (FOB) | Negotiable | transportation | - |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | Negotiable | Shipping time | Negotiable |
- President
- Kyung Mok, Park
- Address
- #306 Pohang Technopark, 601, Jigok-dong, Nam-gu, Pohang-si, Gyeongsangbuk-do, Korea
- Product Category
- Monitoring & Diagnostic Equipment,Pharmaceuticals
- No. of Total Employees
- 1-50
- Company introduction
-
GENOMINE is a company focused on the discovery, development and commercialization of biomarkers and diagnostic kits for early detection of cancers.
GENOMINE has been working on a full range of services to support various proteome-related researches from separation and identification of proteins to analysis of post-translational modifications such as phosphorylation and glycosylation. We have well equipped proteomics core facility and our staff scientists are experienced proteomics professionals.GENOMINE is dedicated to the development of in vitro antibody-based assays for a select group of cancers that are difficult to accurately diagnose without resorting to expensive and invasive methods like biopsies, resulting in numerous, unnecessary procedures at a considerable cost to patients and the health care industry.
Through a long term of collaborative research with medical doctors in cancer hospitals, we successfully discovered novel biomarkers for kidney cancer and developed into immunoassay format to meet clinical test standard. It showed a high sensitivity and specificity in a trial of patients with kidney cancer.
GENOMINE currently has 7 cancer diagnostic programs under development designed to assist with the early detection of cancer, thereby reducing suffering, saving lives and cutting healthcare costs.
- Main Product
Related Products

Covid 19 Detection
AFP/PSA/CEA Rapid Test

PREGABLE Ovulation Pregnancy Tests Kit with APP, SmileReader

Urine Analysis Test Strip Self-Stik Series

R-ligo