ATOBEST PLC-101-B
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- Brand name
- ATOBEST
- Payment Terms
- Negotiable
- Production method
- Negotiable
- Shipping / Lead Time
- Negotiable / Negotiable
- Keyword
- atopic skin care, atopy, moisturizer, biopid
- Category
- Facial Care , Face Cream & Lotion , Skin Care Serum
BPCOSMEDI.CO.,LTD
- Country / Year Established
-
 South Korea
/
South Korea
/
- Business type
- Others
- Verified Certificate
-
13


| Product name | ATOBEST PLC-101-B | Certification | - |
|---|---|---|---|
| Category |
Facial Care
Face Cream & Lotion Skin Care Serum |
Ingredients | - |
| Keyword | atopic skin care , atopy , moisturizer , biopid | Unit Size | - |
| Brand name | ATOBEST | Unit Weigh | - |
| origin | Stock | - | |
| Supply type | - | HS code | 3304991000 |
Product Information
BPCOSMEDI is company for cosmetics distribution of BIOPID.
PLC-101 is the main ingredient of the ATOBEST lineup and this patented ingredient is developed by the BioPid Corporation.
After long term study and research, the company developed a specialized care product effective on atopy, sensitive and super dry skin.
The patented ingredient’s natural phospholipid vitalizes the fat metabolism beneath the skin to help rejuvenate damaged skin.
Unlike the Ceramide-like moisturizing ingredients which are used to temporarily stabilize the skin, PLC-101 will vitalize the skin cells to produce Ceramide on its own to rejuvenate damaged skin.
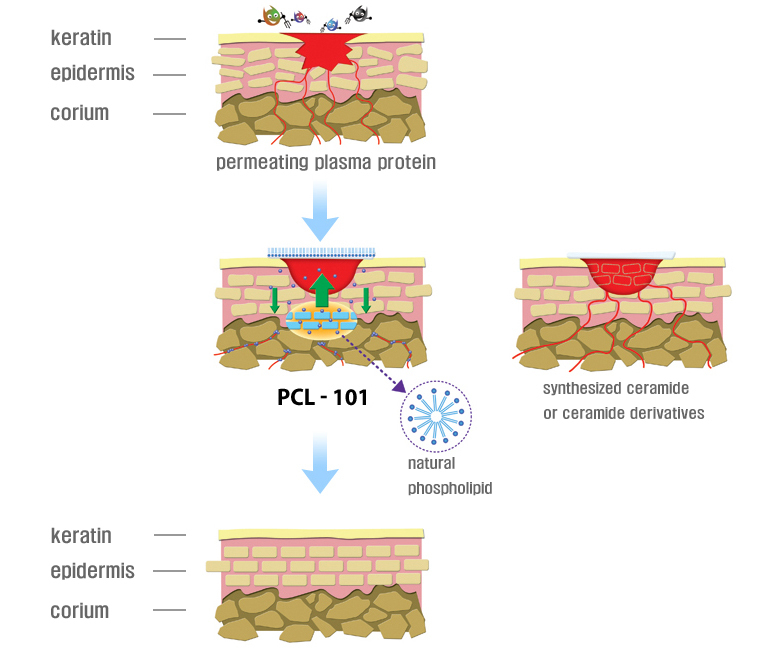
Marketing Concept
1. Safe to use
The patented compound of BioPid, the double saturated phospholipid used in the medication was proven to be safe through clinical trials.
2. Skin rejuvenation
The patented compound double saturated phospholipid helps the skin produce Ceramide on its own to rejuvenate damaged skin.
3. Low skin irritation
By using safe basic materials and a minimum amount of recipes, the product enables a minimum irritation level on atopy and sensitive skin.
4. Technical skills recognized World wide
Registered patent for countries in Korea, Austria, Singapore, China, Japan, Russia India, Hong Kong and EU. Application for patents applied in the U.S, Canada, Brazil.
5. KFDA’s phase 3 Clinical trial completed
Is the first Korean atopy medication to have completed phase 3 clinical trials at 8 university hospitals including Seoul Univ. Hospital, Yeonsei Univ. Severance Hospital, Catholic Univ. St. Mary’s Hospital, and Hanyang Univ. Hospital.
6. KFDA Registered medication
On March 30th, 2011, the medication is the 17th new medication to be listed on KFDA’s new medication registration.
Product Concept
1. Applies direct effect to atopy diseased, sensitive and super dry skin.
2. Forming powerful skin protection layers to create stable conditions under outside irritation.
3. Supplies basic ingredients for Ceramide production.
4. Outstanding moisture keeping ability helps keep skin moist.
5. Very stable against lipid oxidation providing outstanding skin protection.
6. Vitalizing fat metabolism of skin cells allowing smooth and shiny skin to be maintained.
BIOPID Introduction
Biopid Corporation is a bio company that was established in December 2005 for the purposes of research and development of skin disease such as atopic dermatitis, eczema, psoriasis and lung disease such as rhinitis, asthma etc.
Biopid has developed novel and effective materials on atopic dermatitis
It is a composition, *Composition for reducing the exudation of serum proteins* of which a key ingredient is the double-saturated phospholipid developed for treatment of rhinitis, asthma, chronic obstructive pulmonary diseases and noninfectious skin diseases such as atopic dermatitis and the patent registration has been completed in 17 countries of the world.
It was approved phase 2 and 3 clinical trials as a treatment for atopic dermatitis by KFDA, and it was completed clinical trials (three-phase) at 8 Korean university hospitals for the first time in Korea in 2009.
KT&G101 acquired approval of new drugs (KFDA) in nov. 2012
Also, Biopid has completed the development of cosmetics for atopic dermatitis applying the patented ingredient.
Currently, it is developing functional cosmetic for anti-aging and infant.
Biopid has a capital US$3.5M and affiliated companies, company for cosmetics distribution , for atopic medical tourism.
Atopy Medication Development History
Feb. 2005. Application of patent for *Composition for reducing blood protein exudation*
Dec. 2006. Signed transfer of technology contract with KT&G for Atopic dermatitis medication
Aug. 2008. Phase 2 and 3 of clinical trials for Atopic dermatitis medication (KT&G101) approved by Korean Food and Drug Administration
Jan. 2009. Initiation of KT&G 101 phase 2 and 3 clinical trials at 8 Korean university hospitals (Seoul University Hospital, Yeonsei University Severance Hospital, Catholic University of Korea Uijeongbu St. Mary Hospital, Hanyang University Hospital, Chungang University Hospital, Catholic University of Korea Incheon St. Mary Hospital. Hallym University Medical Center, Soonchunyang University Hospital)
Nov. 2009. Completion of Phase 3 clinical trials of Atopic dermatitis medication (KT&G101)
Jul. 2010. Composition of final report of clinical trial of Atopic dermatitis medication (KT&G101)
Mar. 2011. Applied for NDA of Atopic dermatitis medication (KT&G101) at Korean Food & Drug Administration
Nov. 2012 KT&G101 acquired approval of new medicine for Atopic Dermatitis (KFDA)
Patent Status
*Composition for reducing the exudation of serum proteins* patent
- Applicable field : Atopic dermatitis, nasal inflammation, and asthma medication and cosmetics
- Patent registration : Korea (2009), Austria, Singapore, Russia (2010), China, EU (2011), Japan, India, Hong Kong (2012)
- Patent evaluation : U.S, Brazil, Canada
*Compound for accelerating hair growth* applied for patent
- Applicable field : Hair loss prevention / hair growth
- Patent application complete (May, 2010)
- PCT application (May, 2011)
*Patch for the treatment and relief of symptoms of skin diseases with the exudation of serum proteins* applied for patent
- Applicable field : Atopic dermatitis, Eczema, Psoriasis, Erythema, Urticaria
- Patent application complete (Jul, 2010)
- PCT application (Jul, 2011)
ATOBEST PLC-101-B (For body, 30ml : For moderate conditions)
※ Instructions
Apply an optimum amount of “PLC-101B” to the atopy diseased and damaged skin area and to ensure better application, please massage the area for 15-20 seconds (2-3 times a day/ 1 drop for 4cm*4cm skin area recommended)
※ Please use under these circumstances!
1. When skin is tight due to dryness.
2. When skin peels.
3. When skin turns red due to sensitiveness
4. When skin has pimples.
5. When skin is damaged due to outside irritation.
6. When skin is rough and irregular.
7. When skin tone is dark due to irritation
※ Please check when using!
1. When the area is damaged by scratching or unhealthiness, please treat the skin first before using. If used without treatment, the condition may worsen.
2. When the applied area swells up due to a misuse of the product, please stop applying the product and wash the area several times with warm water. This will allow the skin to return to its normal state.
3. Please do not use on your face.
※ Caution
Using the optimum amount will have a significant effect on your skin. Using more than an optimum amount may have adverse effects.
- Product Info Attached File
B2B Trade
| Price (FOB) | Negotiable | transportation | - |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | Negotiable | Shipping time | Negotiable |
- President
- Doo-Jin Park
- Address
- 3F 115-8 Joonggok-dong Kwangjin-gu Seoul Korea
- Product Category
- Face Cream & Lotion,Facial Care
- No. of Total Employees
- 1-50
- Company introduction
-
BPCOSMEDI is company for cosmetics distribution of BIOPID.
Biopid Corporation is a bio company that was established in December 2005 for the purposes of research and development of skin disease such as atopic dermatitis, eczema, psoriasis and lung disease such as rhinitis, asthma etc.
Biopid has developed novel and effective materials on atopic dermatitis
It is a composition, *Composition for reducing the exudation of serum proteins* of which a key ingredient is the double-saturated phospholipid developed for treatment of rhinitis, asthma, chronic obstructive pulmonary diseases and noninfectious skin diseases such as atopic dermatitis and the patent registration has been completed in 17 countries of the world.
It was approved phase 2 and 3 clinical trials as a treatment for atopic dermatitis by KFDA, and it was completed clinical trials (three-phase) at 8 Korean university hospitals for the first time in Korea in 2009.
KT&G101 acquired approval of new drugs (KFDA) in nov. 2012
Also, Biopid has completed the development of cosmetics for atopic dermatitis applying the patented ingredient.
Currently, it is developing functional cosmetic for anti-aging and infant.
Biopid has a capital US$3.5M and affiliated companies, company for cosmetics distribution , for atopic medical tourism.
Atopy Medication Development History
Feb. 2005. Application of patent for *Composition for reducing blood protein exudation*
Dec. 2006. Signed transfer of technology contract with KT&G for Atopic dermatitis medication
Aug. 2008. Phase 2 and 3 of clinical trials for Atopic dermatitis medication (KT&G101) approved by Korean Food and Drug Administration
Jan. 2009. Initiation of KT&G 101 phase 2 and 3 clinical trials at 8 Korean university hospitals (Seoul University Hospital, Yeonsei University Severance Hospital, Catholic University of Korea Uijeongbu St. Mary Hospital, Hanyang University Hospital, Chungang University Hospital, Catholic University of Korea Incheon St. Mary Hospital. Hallym University Medical Center, Soonchunyang University Hospital)
Nov. 2009. Completion of Phase 3 clinical trials of Atopic dermatitis medication (KT&G101)
Jul. 2010. Composition of final report of clinical trial of Atopic dermatitis medication (KT&G101)
Mar. 2011. Applied for NDA of Atopic dermatitis medication (KT&G101) at Korean Food & Drug Administration
Nov. 2012 KT&G101 acquired approval of new medicine for Atopic Dermatitis (KFDA)
Patent Status
*Composition for reducing the exudation of serum proteins* patent
- Applicable field : Atopic dermatitis, nasal inflammation, and asthma medication and cosmetics
- Patent registration : Korea (2009), Austria, Singapore, Russia (2010), China, EU (2011), Japan, India, Hong Kong (2012)
- Patent evaluation : U.S, Brazil, Canada
*Compound for accelerating hair growth* applied for patent
- Applicable field : Hair loss prevention / hair growth
- Patent application complete (May, 2010)
- PCT application (May, 2011)
*Patch for the treatment and relief of symptoms of skin diseases with the exudation of serum proteins* applied for patent
- Applicable field : Atopic dermatitis, Eczema, Psoriasis, Erythema, Urticaria
- Patent application complete (Jul, 2010)
- PCT application (Jul, 2011)
- Main Product












































_2.jpg)
