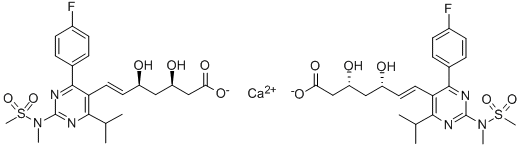
Rosuvastatin calcium CAS No.: 147098-20-2
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- Brand name
- 366
- Payment Terms
- Negotiable
- Production method
- Negotiable
- Shipping / Lead Time
- Negotiable / Negotiable
- Category
- Pharmaceutical Intermediates
366 Pharma (Nanjing) Co., Ltd.
- Country / Year Established
-
 China
/
China
/
- Business type
- Others
- Verified Certificate
-
14

| Product name | Rosuvastatin calcium CAS No.: 147098-20-2 | Certification | - |
|---|---|---|---|
| Category | Pharmaceutical Intermediates | Ingredients | - |
| Keyword | cardiovascular , rosuvastatin , rosuvastatin calcium , 147098-20-2 | Unit Size | - |
| Brand name | 366 | Unit Weigh | - |
| origin | Stock | - | |
| Supply type | - | HS code | - |
Product Information
|
Physical and Chemical Properties |
|||
|
Common Name |
Rosuvastatin calcium |
||
|
Chemical Name |
|
||
|
Molecular Formula |
C44H54CaF2N6O12S2 |
Molecular Weight |
1001.14 |
|
CAS No. |
147098-20-2 |
EINECS No. |
|
|
Product Specification |
|||
|
Appearance |
White or yellow powder |
||
|
Purity |
98.0% |
Shelf life |
2 years |
|
Quality Standard |
In-house |
||
|
Certification |
In process of applying for GMP and ISO 9001 |
||
|
Product Description |
Rosuvastatin is a member of the drug class of statins, used to treat high cholesterol and related conditions, and to prevent cardiovascular disease.
Rosuvastatin was initially developed by Shionogi in the late 1980s; the original development number is S-4522. In June, 1998, the development, market and sales rights were in-licensed to AstraZeneca. Rosuvastatin was approved for the first time in Holland on November 7, 2002, and then was approved in Canada in Feb, 2003. Approval in the United States by the FDA came on August 12, 2003.
Rosuvastatin is a competitive inhibitor of the enzyme HMG-CoA reductase, having a mechanism of action similar to that of other statins. Its approximate elimination half life is 19 hours and its time to peak plasma concentration is reached in 3–5 hours following oral administration.
We manufacture and supply APIs and relative intermediates of Statin series for cardiovascular disease, including Pitavastatin, Rosuvastatin, Atorvastatin and Fluvastatin.
TECHNICAL DATA SHEET
|
Item |
Specification |
|
Appearance |
White to yellow powder |
|
Identification |
IR-Spectrum |
|
UV |
|
|
Calcium content |
3.5%-4.5% |
|
Water |
≤5.0% |
|
Specific Rotation |
14.0-20.0 |
|
Heavy Metals |
≤0.002% |
|
Relative Substance |
Diastereomer ≤0.30% |
|
Rosuvastatin lactone ≤0.20% |
|
|
Diene ≤0.30% |
|
|
Unknown single impurity ≤0.20% |
|
|
Total impurity ≤1.0% |
|
|
Assay (On dry basis) |
98.0-102.0% |
|
Packaging & Shipping |
|
|
Packaging Spec. |
1kg/bag, 25kg/drum |
|
Shipping Mode |
Air or express |
|
Loading Port |
Nanjing, Shanghai or other China main ports |
|
Delivery Time |
Depending upon the order volume |
|
Payment |
|
|
Payment Mode |
T/T, L/C, D/P, D/A and others |
Note: For small value order, we always require 100% T/T in advance as payment term.
B2B Trade
| Price (FOB) | Negotiable | transportation | - |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | Negotiable | Shipping time | Negotiable |
- President
- Betty Bi
- Address
- 80 Yunlongshang Road, Nanjing, Jiangsu 210019, China
- Product Category
- Food Additives,Pharmaceutical Intermediates
- Main Product
Related Products
Hydrocoralliaire- Klonopin- Lexapro
Phenylbutazone
Betamethasone 17-valerate

Limonin

120/80 SYMBIOSAL _ antihypertensive salt