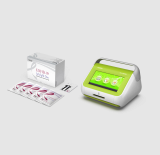
FREND System
Microfluidic immunofluorescence analyzer
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- South Korea
- Payment Terms
- Others
- Production method
- OBM
- Shipping / Lead Time
- Negotiable / Negotiable
- Category
- Medical Devices
NanoEnTek Inc.
- Recent Visit
- Dec 20, 2024
- Country / Year Established
-
 South Korea
/
2000
South Korea
/
2000
- Business type
- Manufacturer
- Verified Certificate
-
16



| Product name | FREND System | Certification | - |
|---|---|---|---|
| Category | Medical Devices | Material | - |
| Keyword | in vitro diagnostic , poct , in vitro diagnostic test , immunoassay | Unit Size | - |
| Brand name | - | Unit Weigh | - |
| origin | South Korea | Stock | 0 |
| Supply type | OBM | HS code | - |
Product Information
- Accurate & compact/Easy-to-use
- Rapid and quantitative immunoassay
- Small sample volume analysis
- Innovative microchip and microfluidic technologies
- Flexible platform for multiple tests

One drop! 3 minutes!
With a simple 3 step operation and a wide range of disease markers, the system supports quick decision-making.
It is expected to increase patients’ satisfaction and a hospital’s profitability by improving the effectiveness of the test.

Microfluidics Technology Leader
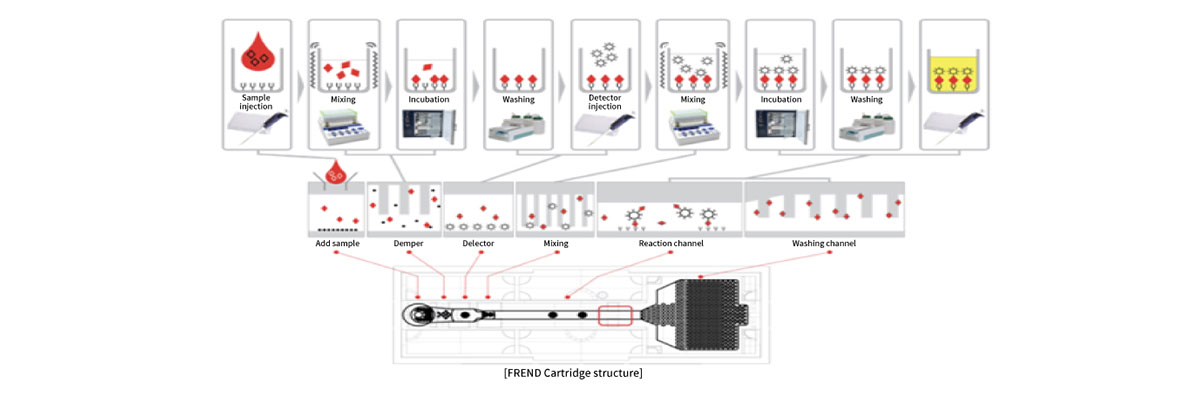
The FREND™ System is an in vitro diagnostic device that uses microfluidics technology and immunofluorescent techniques.
Disposable plastic microfluid chip continuously mixes, reacts, separates, and quantitatively analyzes on the chip.
This is a source technology that is the foundation for bioengineering experiments and point-of-care test (POCT).
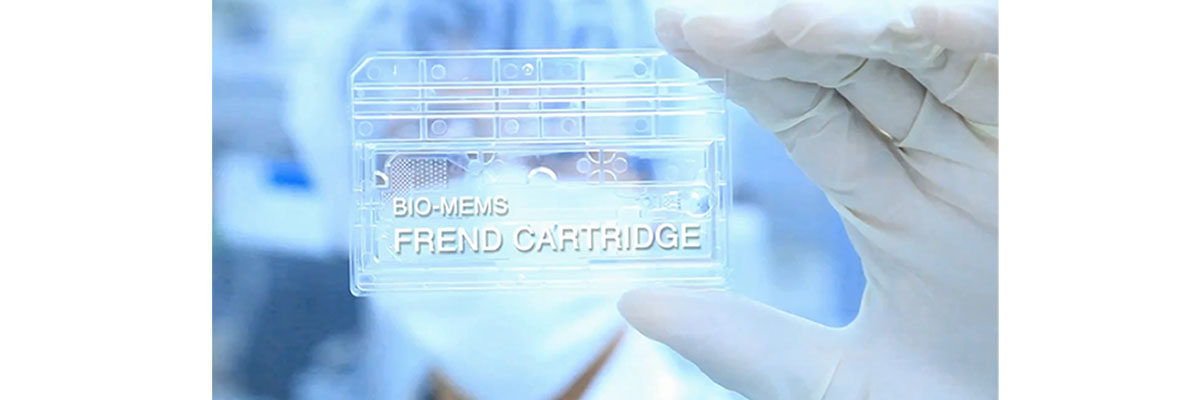
Excellent Quality Control
Use the QC cartridge to quickly check the integrity of the system. The FREND™ System cleared 510(k) as the in vitro diagnostic (IVD).
The code chip significantly reduces possible errors in between test values and LOT variation, hence provides more accurate results.

Superior Accuracy
The FREND™ System received FDA 510(k) clearance as the in-vitro diagnostics
which provides a high correlation with large machinery and provides accurate results.

FDA 510(k) clearance
PSA Plus, TSH, Free T4, Testosterone and Vitamin D cleared from FDA 510(k).
FREND™ System provides a variety of other inspection items, which can be tested for a total of 12 different types.
including BNP, Troponin I, Cardiac Triple, PCT, Total T3, and Thyroid Duo.

B2B Trade
| Price (FOB) | Negotiable | transportation | Negotiation Other |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | Others | Shipping time | Negotiable |
- President
- Chanil Chung
- Address
- Guro-gu, Guro-dong,235-2, Guro-gu, Seoul, Korea
- Product Category
- Medical Devices
- Year Established
- 2000
- No. of Total Employees
- 101-500
- Company introduction
-
- Main Markets
-
 Germany
Germany
 Italy
Italy
 Japan
Japan
 Taiwan
Taiwan
 U. Kingdom
U. Kingdom
- Main Product