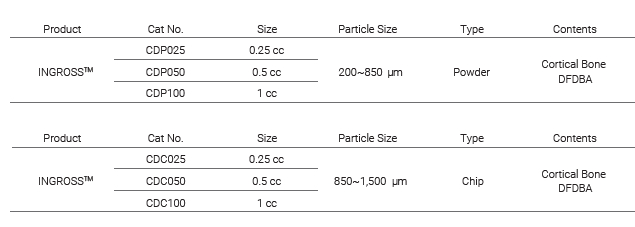
INGROSS_Cortical Bone & DFDBA - Powder and Chip
INGROSS™: FDBA/DFDBA cortical bone mix for optimal osteoinduction and osteoconduction in curved syringe for easy application
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- South Korea
- Brand name
- INGROSS™
- Payment Terms
- T/T
- Production method
- Available
- Shipping / Lead Time
- Negotiable / Negotiable
- Keyword
- allograft, bone graft, fdba/dfdba
- Category
- Dental Consumables , Other Medical Consumables

HANS BIOMED CORP.
- Verified Certificate
-
17



| Product name | INGROSS_Cortical Bone & DFDBA - Powder and Chip | Certification | - |
|---|---|---|---|
| Category |
Dental Consumables
Other Medical Consumables |
Material | - |
| Keyword | allograft , bone graft , fdba/dfdba | Unit Size | - |
| Brand name | INGROSS™ | Unit Weigh | 0 mg |
| origin | South Korea | Stock | 1 |
| Supply type | Available | HS code | - |
Product Information
INGROSS™ is an innovative bone graft material,
combining the benefits of FDBA (Freeze Dried Bone Allograft) and DFDBA (Demineralized Freeze Dried Bone Allograft).
This unique mixture preserves growth factors and minerals essential for bone regeneration,
resulting in superior osteoconduction and osteoinduction capabilities.
The FDBA component, derived from cortical and cancellous bone, maintains its natural mineral content,
providing excellent osteoconductive properties.
The DFDBA portion, created by demineralizing cortical bone, enhances osteoinductive potential.
This synergistic combination promotes rapid integration with the recipient site,
facilitating healthy and robust bone regeneration through both osteoconduction and osteoinduction processes.
B2B Trade
| Price (FOB) | Negotiable | transportation | Air Transportation,Express,Negotiation Other,Ocean Shipping |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | T/T | Shipping time | Negotiable |

- President
- HWANG, HO-CHAN
- Address
- Chongyi-ro 8 gil 7, Songpa-gu, Seoul, Korea
- Product Category
- Medical Consumables,Medical Devices
- Year Established
- 1999
- No. of Total Employees
- 101-500
- Company introduction
-
HansBiomed Corporation, established in 1999, is a global leader in tissue engineering and medical devices,
focusing on innovative aesthetic solutions.
Present in over 100 countries, HansBiomed has become a trusted partner in the aesthetic industry.
As a pioneer in the field, the company established Korea's first tissue bank in 2005 and is FDA-registered.
With a strong emphasis on R&D and innovation, HansBiomed has achieved consistent growth,
operating globally and exporting high-quality tissue transplants and medical products worldwide.
- Main Markets
-
 China
China
 Colombia
Colombia
 Russia
Russia
 U.S.A
U.S.A
- Main Product
- Attached File



































 South Korea
South Korea



_2.png)
_2.jpg)


