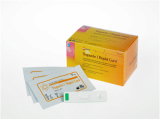
GreenCare FLU2 Influenza A&B Ag
Influenza A&B Ag Test
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- South Korea
- Payment Terms
- T/T
- Production method
- Available,OBM,ODM,OEM
- Shipping / Lead Time
- Negotiable / Negotiable

GC Medical Science CORP.
- Recent Visit
- Jan 20, 2025
- Country / Year Established
-
 South Korea
/
2003
South Korea
/
2003
- Business type
- Manufacturer
- Verified Certificate
-
17


| Product name | GreenCare FLU2 Influenza A&B Ag | Certification | - |
|---|---|---|---|
| Category |
Medical Test Kit
Laryngoscope Other Monitoring & Diagnostic Equipment |
Material | - |
| Keyword | influenza , rapid , covid | Unit Size | - |
| Brand name | - | Unit Weigh | - |
| origin | South Korea | Stock | 200 |
| Supply type | Available,OBM,ODM,OEM | HS code | - |
Product Information
The GreenCare FLU2 Influenza A&B Ag test is an in vitro diagnostic medical device designed for rapid and qualitative detection of influenza A and B virus antigens. Utilizing immunochromatography, this test provides a swift analysis of nasopharyngeal samples to identify the presence of influenza virus antigens.
The test is specifically engineered to deliver reliable results for the detection of influenza A and B, assisting in the effective diagnosis and management of influenza infections.
B2B Trade
| Price (FOB) | Negotiable | transportation | Air Transportation,Ocean Shipping |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | T/T | Shipping time | Negotiable |

GC Medical Science CORP.
- Country / Year Established
-
 South Korea
/
2003
South Korea
/
2003
- Recent Visit
- Jan 20, 2025
- Business type
- Manufacturer
-
17


- President
- Seung-Sam Seo
- Address
- Yongin-si, Guseong-eup, Bojeong-ri,#303, Giheung-gu, Yongin-si, Gyeonggi-do, Korea
- Product Category
- Glucose Meter,Medical Test Kit,Multi-Parameter Monitor,Other Health Care Products,Other Monitoring & Diagnostic Equipment
- Year Established
- 2003
- No. of Total Employees
- 101-500
- Company introduction
-
We started as a blood type diagnosis business of Green Cross in 1972 and established it in 2003 based on technology and know-how in the field of medical devices and diagnostic reagents. The business currently in operation consists of diagnostic reagents, hemodialysis fluids, and chronic disease projects.
We are a leading diagnostic company in future industries with global performance and quality systems with international certification such as CE as well as the Ministry of Food and Drug Safety in Korea. In particular, in the case of overseas businesses, we focus on field diagnostic products and export measuring devices and reagents such as glycated hemoglobin, blood sugar, and hemoglobin developed based on differentiated patent technology to more than 90 countries around the world.
- Main Product
- Attached File



































_2.jpg)
_2.jpg)
_2.jpg)