Rejeunesse Fine Deep Shape
1.1ml x 1 syringe HA 24mg/mL, Lido 0.3%
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- South Korea
- Payment Terms
- Others,T/T
- Production method
- Available
- Shipping / Lead Time
- Negotiable / Negotiable
- Keyword
- botox, dermal filler, filler, rejeunesse

Starpharmtec Co., Ltd.
- Membership
- PRO
- Recent Visit
- Jan 09, 2025
- Country / Year Established
-
 South Korea
/
2020
South Korea
/
2020
- Business type
- Distributor
- Verified Certificate
-
1

| Product name | Rejeunesse Fine Deep Shape | Certification | - |
|---|---|---|---|
| Category |
Other Beauty Products
Facial Care Medical Consumables Pharmaceuticals Other Beauty Appliance |
Ingredients | HYALURONIC ACID |
| Keyword | botox , dermal filler , filler , rejeunesse | Unit Size | - |
| Brand name | - | Unit Weigh | - |
| origin | South Korea | Stock | 10000 |
| Supply type | Available | HS code | 3304991000 |
Product Information
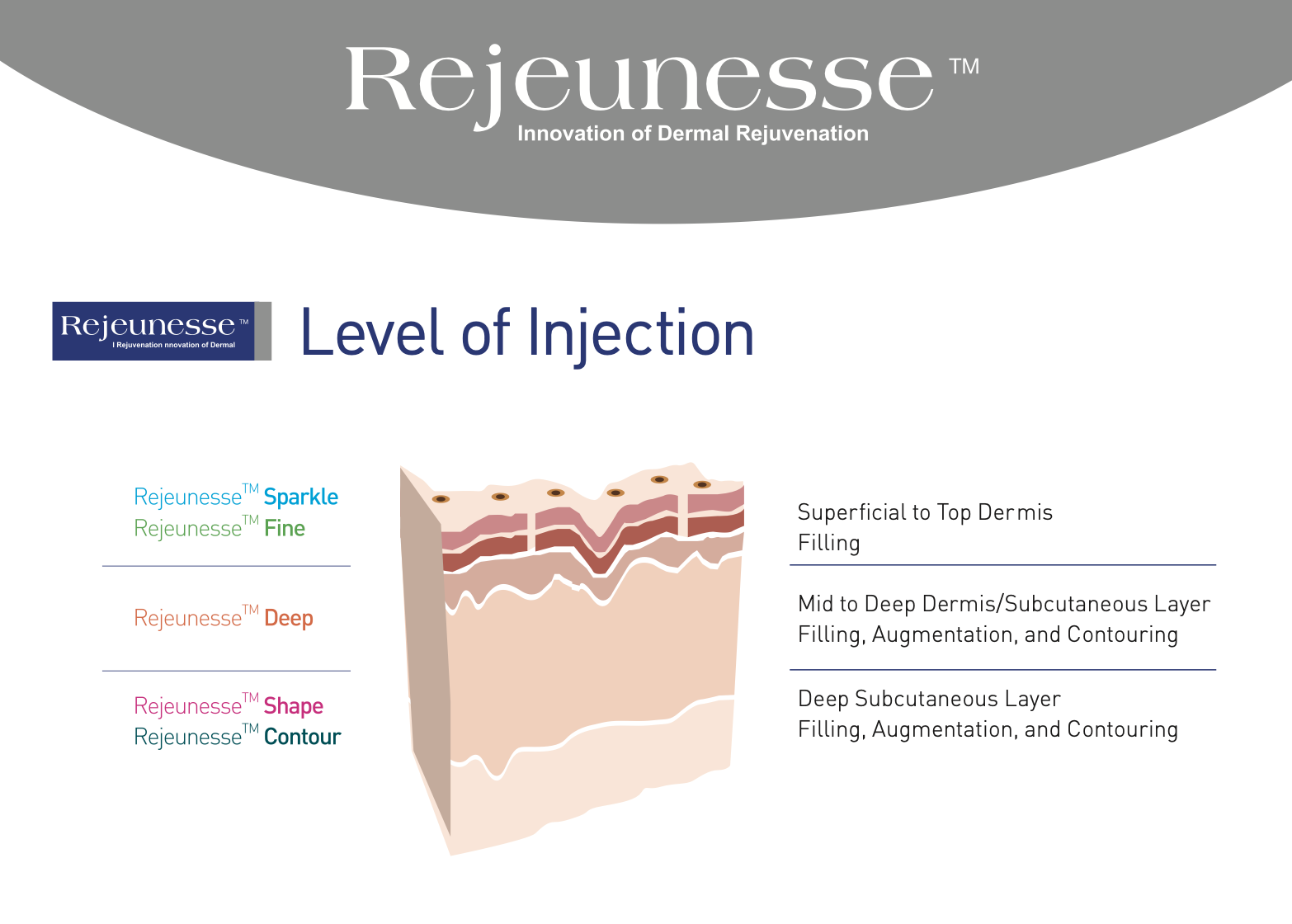
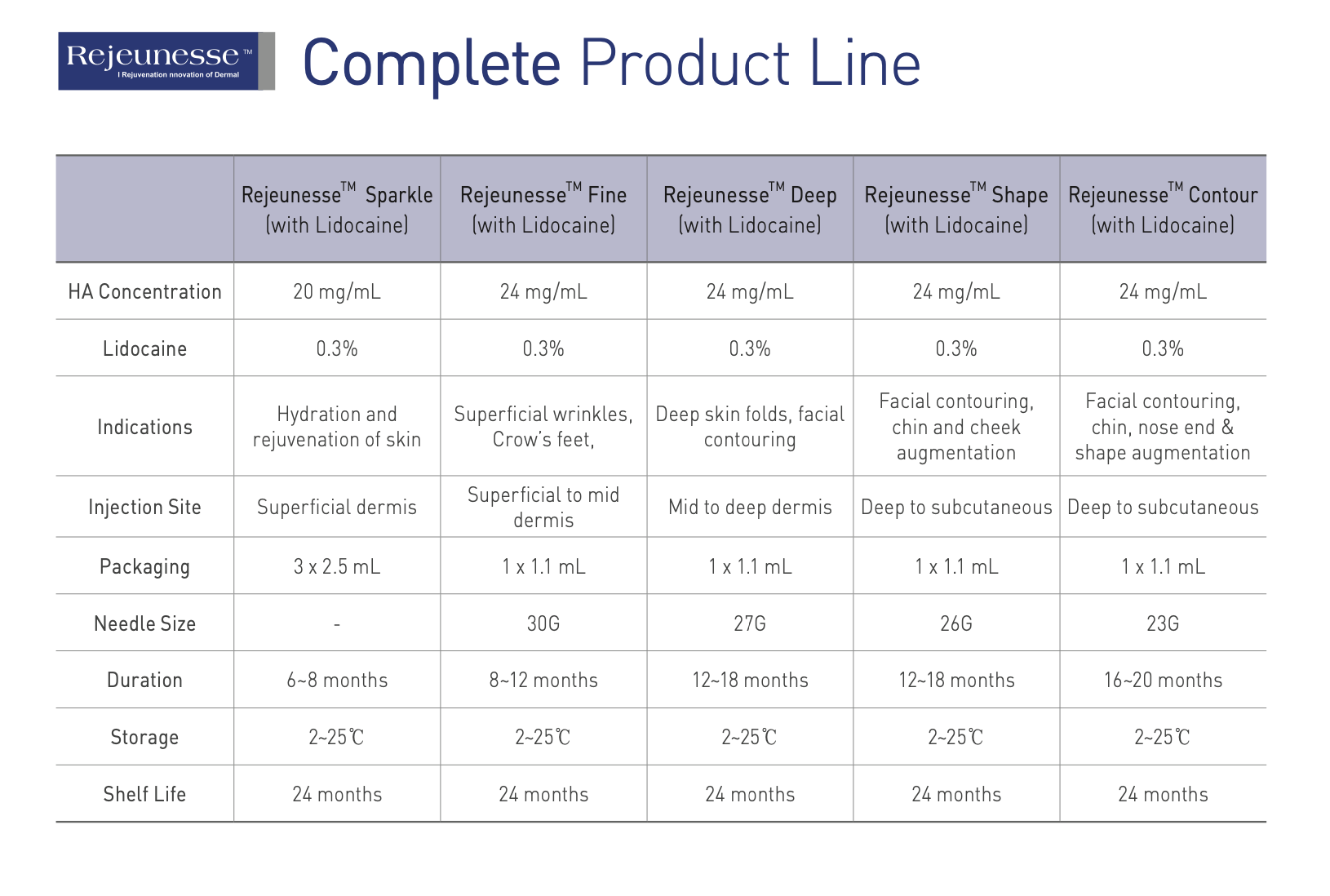
Rejeunesse Fine | Deep | Shape
1.1mL x 1syringe | HA 24mg/mL | Lido caine 0.3%
Rejeunesse™ is a non-animal based dermal filler contains hyaluronic acid (HA) at a concentration of 24mg/ml, with the addition of Lido caine at a concentration of 0.3%
Rejeunesse Fine : Needles 30G x 2pcs
Rejeunesse Deep : Needles 27G x 2pcs
Rejeunesse Shape : Needles 26G x 2pcs
Rejeunesse™ with lido caine is a sterile, transparent, pyrogen-free, viscoelastic gel of 100% crosslinked hyaluronic acid of non-animal origin containing 0.3% lido caine hydrochloride for treatment of wrinkles and folds. The lido caine provides the patient’s pain reducing effect during treatment.
• Safety : Rejeunesse™ is non-animal based, crosslinked dermal filler using BDDE for linking agent. Rejeunesse™ product is obtained through our unique UPHEC technology, making it safe and non carcinogenic for humans. Rejeunesse™ contains less than 1.2EU/mL of endotoxins with no BDDE residue detected and it exceeds the highest level of HA purity. Rejeunesse™ is completely free of all animal products thus reducing the risk of an immunogenic reaction.
• Long-lasting : Once injected, HA is gradually broken and absorbed into the body, which is why how strongly HA is crosslinked is crucial to the longevity of the dermal fillers. With unique crosslinking technology, Rejeunesse™ has longevity over 12months.
• Efficacy : Rejeunesse™ offers visible results immediately and stable volume effect with minimized swelling after application.
• Treatment without pain : Rejeunesse™ has optimized the product to minimize the pain during and/or post injection for patients. Monophasic Rejeunesse™ enables smoothest and stable injection during procedure by maintaining consistent injection force. Especially, Rejeunesse™ Lido caine series which combines 0.3% of Lido caine (local an esthetic) minimizes injection pain during the procedure. Thus, it allows patients to experience the most comfortable, almost painless, procedure of dermal fillers.
Linda Kim | Manager
Whatsapp | Instagram | Website
+82 1063973026
Starpharmtec INC.
B2B Trade
| Price (FOB) | Negotiable | transportation | Air Transportation,Express,Land Transportation,Negotiation Other,Ocean Shipping |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | Others,T/T | Shipping time | Negotiable |

- President
- Kwanghee Seo
- Address
- #201, J bldg., 271-6, Dapsimni-ro, Dongdaemun-gu, Seoul, Korea
- Product Category
- Other Beauty Products,Other Medical Consumables,Other Personal Care
- Year Established
- 2020
- No. of Total Employees
- 1-50
- Company introduction
-
Starpharmtec Co., ltd.(Starpharmtec) is a Pharmaceutical Company founded in May
2015, with a wide array of premium quality products from Korea and other advanced countries.
- The first principal of Starpharmtec is Star Pharmaceutical Company(Korea),
one of the leading medicines and toxin distributing companies in Korea.
- In 2020, Star Pharmaceutical Company established Starpharmtec Co., Ltd. for export
and import professionally.
- Starpharmtec setup company in USA, namely Bluciel Inc.
- Export to 27 countries in 2021.
- Main Markets
-
 Indonesia
Indonesia
 Poland
Poland
 U. Kingdom
U. Kingdom
 U.A.E.
U.A.E.
 U.S.A
U.S.A
- Main Product
- Attached File








































__2.png)
_CE_Approved_2.png)



