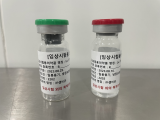
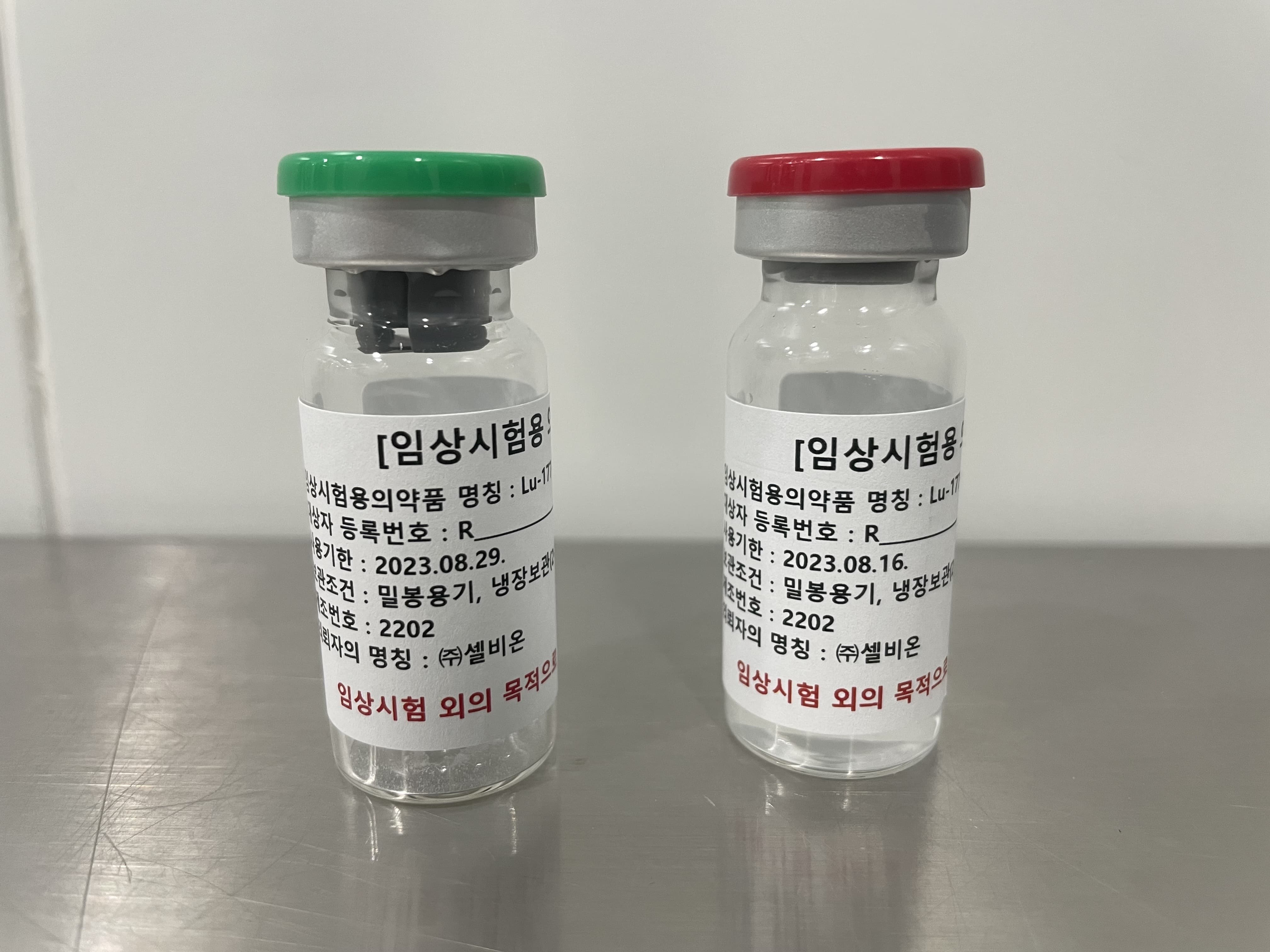
PSMA-DGUL
Radioligand Therapy for Prostate Cancer
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- South Korea
- Brand name
- PSMA-DGUL
- Payment Terms
- Others
- Production method
- Available
- Shipping / Lead Time
- Negotiable / Negotiable
- Keyword
- prostate, medicine & drugs
- Category
- Medicines
CELLBION CO.,LTD.
- Verified Certificate
-
1


| Product name | PSMA-DGUL | Certification | - |
|---|---|---|---|
| Category | Medicines | Material | - |
| Keyword | prostate , medicine & drugs | Unit Size | - |
| Brand name | PSMA-DGUL | Unit Weigh | - |
| origin | South Korea | Stock | 1 |
| Supply type | Available | HS code | - |
Product Information
Lu-177 DGUL is a radiopharmaceutical agent specifically designed to target and treat prostate cancer cells by exploiting the overexpression of the prostate-specific membrane antigen (PSMA) on their surface. PSMA, a type II transmembrane glycoprotein, is predominantly found in prostate cancer cells, while its presence in normal tissues is relatively limited. This differential expression makes PSMA an attractive target for the development of targeted therapies, such as Lu-177-DGUL, for prostate cancer.
Lu-177-DGUL is comprised of three essential components: the novel DGUL molecule, a linker, and the radionuclide Lutetium-177 (Lu-177).
The DGUL molecule serves as a targeting moiety, selectively binding to PSMA with high affinity. This selective binding ensures that the radiopharmaceutical agent primarily targets prostate cancer cells while sparing healthy cells.
The linker is a chemical component that connects the DGUL molecule to the radionuclide, Lu-177. The linker plays a vital role in maintaining the stability of the radiolabeled compound and ensuring that the radionuclide remains attached to the targeting moiety until it reaches the desired site of action. The choice of an appropriate linker is essential, as it can influence the pharmacokinetics, biodistribution, and efficacy of the radiopharmaceutical.
Lu-177, a radioactive isotope of Lutetium, is chosen as the radionuclide for Lu-177-DGUL due to its favorable properties. It emits beta particles, which have a relatively short range and moderate energy, allowing them to cause DNA damage and cell death in the targeted cancer cells. Moreover, Lu-177 has a half-life of approximately 6.7 days, which provides sufficient time for the radiopharmaceutical agent to localize in the tumor and exert its therapeutic effect.
The design of Lu-177-DGUL ensures rapid secretion from the body, reducing the duration of exposure to radiation and minimizing potential toxicities and side effects. This is particularly important for patients who may be more sensitive to radiation, such as the elderly or those with pre-existing medical conditions.
Upon administration, Lu-177-DGUL circulates through the bloodstream and selectively binds to PSMA on the surface of prostate cancer cells. The radiolabeled compound is then internalized, and the Lu-177 emits beta radiation, causing DNA damage and subsequent cell death. This targeted approach enables a more precise and effective treatment, minimizing damage to healthy tissue and reducing the side effects commonly associated with traditional cancer therapies such as chemotherapy and radiation therapy.
DGUL, a novel radiopharmaceutical agent, is specifically designed to target and treat prostate cancer cells by exploiting their overexpression of the prostate-specific membrane antigen (PSMA). PSMA is a type II transmembrane glycoprotein that is highly overexpressed on the surface of prostate cancer cells, while its expression in normal tissues is limited. This characteristic makes PSMA an ideal target for the development of targeted therapies for prostate cancer.
DGUL consists of three main components: a targeting moiety, a linker, and a radionuclide. The targeting moiety is a small molecule or peptide that specifically binds to PSMA with high affinity. This selective binding ensures that the radiopharmaceutical agent primarily targets prostate cancer cells, leaving healthy cells largely unaffected.
The linker is a chemical component that connects the targeting moiety to the radionuclide. It plays a crucial role in maintaining the stability of the radiolabeled compound and ensuring that the radionuclide remains attached to the targeting moiety until it reaches the desired site of action. The choice of an appropriate linker is essential, as it can affect the pharmacokinetics, biodistribution, and efficacy of the radiopharmaceutical.
The radionuclide is the radioactive component of DGUL that emits radiation upon decay, ultimately causing DNA damage and cell death in the targeted cancer cells. Two common radionuclides used in DGUL are Lutetium-177 (Lu-177) and Actinium-225 (Ac-225). Lu-177 emits beta particles, which have a relatively short range, whereas Ac-225 emits alpha particles, which possess higher energy and can cause greater DNA damage. The choice of radionuclide depends on the specific clinical application and desired treatment outcomes.
Upon administration, DGUL travels through the bloodstream and selectively binds to PSMA on the surface of prostate cancer cells. Once bound, the radiolabeled compound is internalized into the cancer cell, where the radionuclide emits radiation that causes DNA damage, ultimately leading to cell death. This targeted approach allows for a more precise and effective treatment, minimizing damage to healthy tissue and reducing the side effects commonly associated with traditional cancer therapies such as chemotherapy and radiation therapy.
DGUL has shown promise in clinical trials as a targeted therapy for patients with advanced, metastatic, or treatment-resistant prostate cancer. Its ability to deliver a potent dose of radiation directly to cancer cells while sparing healthy tissue makes it an attractive option for patients who have exhausted other treatment options or who are not candidates for surgery or radiation therapy.
In conclusion, DGUL represents a promising targeted approach to treating prostate cancer. By selectively targeting PSMA overexpressed on cancer cells, DGUL minimizes damage to healthy tissue, potentially reducing side effects and improving the quality of life for patients undergoing cancer treatment. The combination of a targeting moiety, linker, and radionuclide allows for the precise delivery of radiation to cancer cells, ultimately leading to DNA damage and cell death. As research continues, it is hoped that DGUL and similar targeted radiopharmaceuticals will offer new therapeutic options for patients with prostate cancer and other malignancies.
DGUL, a novel radioligand therapy, has been specifically designed to offer enhanced selectivity for Prostate-Specific Membrane Antigen (PSMA), a transmembrane protein that is highly expressed in prostate cancer cells. This high degree of selectivity ensures accurate targeting of prostate cancer cells, while minimizing damage to healthy cells. DGUL's unique properties are crucial in addressing the significant unmet medical needs of patients suffering from metastatic castration-resistant prostate cancer (mCRPC), a highly aggressive form of the disease that currently has limited treatment options.
One of the notable features of DGUL is its rapid renal excretion, which effectively reduces the side effects associated with unbound radioligand agents. This is particularly important because the accumulation of unbound radioligand agents in healthy tissues can lead to significant toxicity and adverse effects. By promoting rapid clearance of the unbound agents from the body, DGUL minimizes the potential harm to non-target tissues and maximizes patient safety.
The accelerated renal excretion of DGUL also allows for the administration of higher millicurie (mCi) doses of Lutetium-177 (Lu-177), a radioisotope commonly used in radioligand therapy. The higher doses of Lu-177 are believed to potentially increase the therapeutic efficacy of the treatment by delivering a more potent radiation dose to the targeted cancer cells. This increased potency may result in better tumor control and improved clinical outcomes for patients.
DGUL has recently demonstrated promising results in Phase 2 clinical trials, showcasing its potential to be a highly effective treatment option for patients with mCRPC. The positive outcomes from these trials have led to the orphan drug designation for DGUL in Korea, which highlights the importance of this novel therapy in addressing unmet medical needs in the field of prostate cancer treatment.
The orphan drug designation for DGUL in Korea is a significant milestone, as it provides various benefits such as faster regulatory approvals, reduced fees, and market exclusivity. This designation is expected to accelerate the development and commercialization of DGUL, ultimately benefiting patients suffering from mCRPC who currently have limited treatment options.
In summary, DGUL is a promising radioligand therapy that combines enhanced selectivity for PSMA with rapid renal excretion to minimize side effects and maximize patient safety. The positive Phase 2 clinical trial results and orphan drug designation in Korea underscore its potential to address the unmet medical needs of patients with mCRPC. By enabling the administration of higher mCi of Lu-177, DGUL may offer improved therapeutic efficacy and clinical outcomes for patients suffering from this aggressive form of prostate cancer.
- Product Info Attached File
B2B Trade
| Price (FOB) | Negotiable | transportation | Negotiation Other |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | Others | Shipping time | Negotiable |
- President
- KIM GWON
- Address
- 6F, 103, Daehak-ro, Jongno-gu, Seoul, Korea
- Product Category
- Medicines
- Year Established
- 2010
- No. of Total Employees
- 1-50
- Company introduction
-
- Main Markets
-
 Germany
Germany
 Switzerland
Switzerland
 U. Kingdom
U. Kingdom
 U.S.A
U.S.A
- Main Product
- Attached File
Related Products
_2.jpg)
VINIX ORAL DISSOLVING FILM 50mg,100mg(SILDENAFIL 50mg, 100mg)
NOVA PMC
Lactomin
NOVA SlimBS
_2.jpg)
NIPEP-IBD (peptide for IBD treatment)


































 South Korea
South Korea