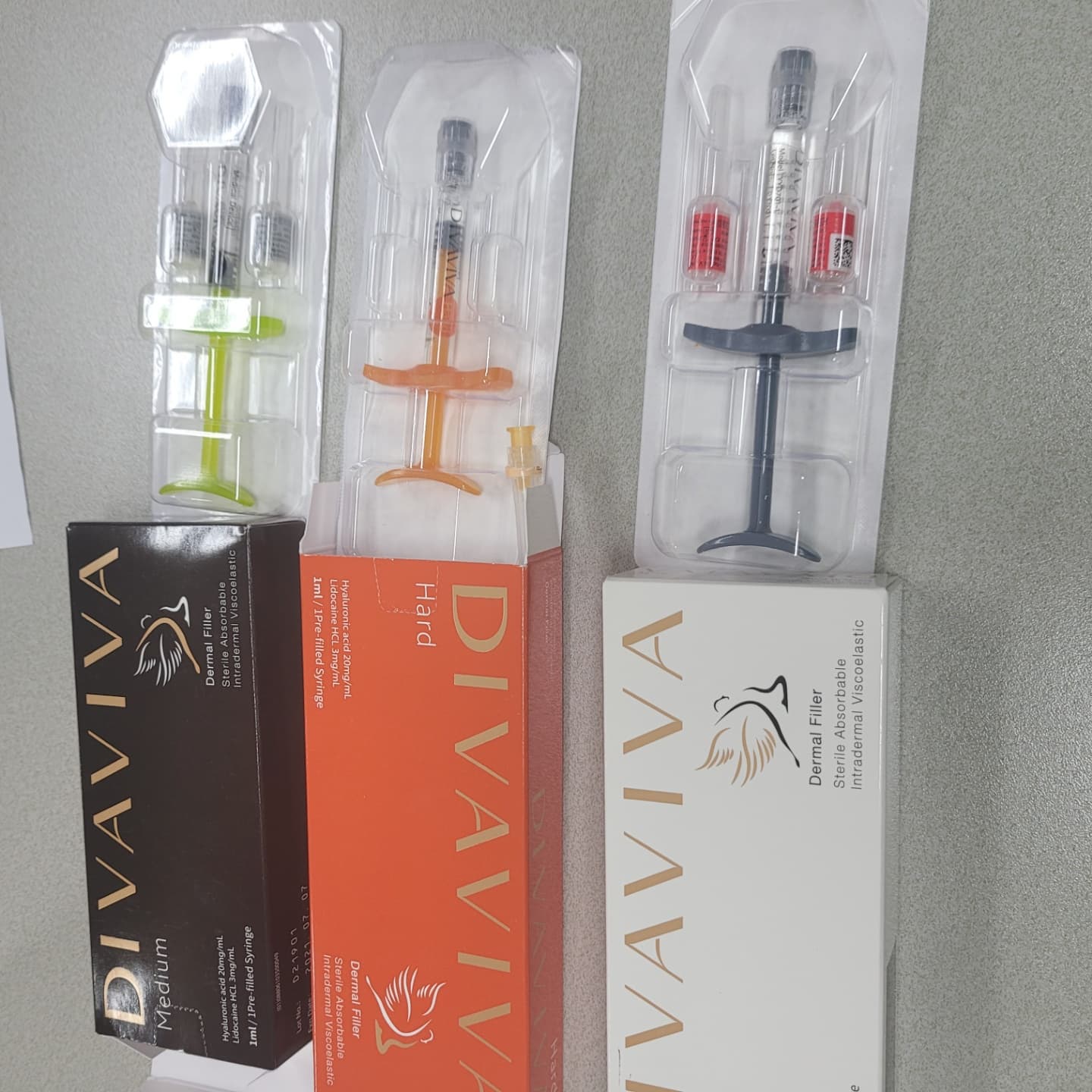
DIVA VIVA, HA Dermal Filler, Face filler, botulax, medical device, medical product
Dermal FILLER, Beauty medical,
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- South Korea
- Brand name
- DIVAVIVA
- Payment Terms
- Others,T/T
- Production method
- Available
- Shipping / Lead Time
- Negotiable / Negotiable
Athena International Co., Ltd.
- Membership
- PRO
- Recent Visit
- Nov 13, 2024
- Country / Year Established
-
 South Korea
/
2022
South Korea
/
2022
- Business type
- Trading Company
- Verified Certificate
-
2


| Product name | DIVA VIVA, HA Dermal Filler, Face filler, botulax, medical device, medical product | Certification | - |
|---|---|---|---|
| Category |
Medical Devices
Medical Consumables Medical Centrifuge Medical Face Mask Other Medical Consumables |
Material | - |
| Keyword | dermal filler , filler , medical device , medical product , face filler , ha dermal filler , botulax , diva viva | Unit Size | - |
| Brand name | DIVAVIVA | Unit Weigh | 0 kg |
| origin | South Korea | Stock | 10000 |
| Supply type | Available | HS code | - |
Product Information
1. Product Name : DIVA VIVA (HARD, Medium, SOFT ) 2. Ingredient : Cross-linked HA Sodium (20mg) 0.3% Lido 3. Advantage : Nice inject-ability with low-injection force. Natural feature with low swelling ratio.Long duration time. 4. Classification : Medical device 5. Formulation : 1ml Pre-filled Syringe as injection formulation.
6. MFDS Approved Soft/Medium/Hard
DIVAVIA includes lidocaine & cross-linked Hyaluronic acid are for mid-to-deep injection into the facial tissue for the correction of moderate to severe facial wrinkles & folds, such as nasolabial fold.

Product(Code) Name : DIVAVIVA (Soft, Medium and Hard)
Classification : Medical device
Main Ingredient : Cross-linked HA Sodium (20mg) with Lido (0.3%)
Formulation : 1ml Pre-filled Syringe as injection formulation
B2B Trade
| Price (FOB) | Negotiable | transportation | Air Transportation,Express,Land Transportation,Negotiation Other,Ocean Shipping |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | Others,T/T | Shipping time | Negotiable |
- President
- Stella Chung
- Address
- #302, Shelton Building , 21, Jong-ro 44-gil, Jongno-gu, Seoul, Korea
- Product Category
- Medical Devices,Medical Test Kit,Other Medical Consumables
- Year Established
- 2022
- No. of Total Employees
- 1-50
- Company introduction
- Main Markets
-
 Japan
Japan
 Malaysia
Malaysia
 Taiwan
Taiwan
 U.A.E.
U.A.E.
 U.S.A
U.S.A
 Viet Nam
Viet Nam
- Main Product
Related Products

INCYTO Needle

Hairview

Dental Implant Ratchet Hex Drivers

Implant system (SLA surface)

Dental Instruments Manufacturer Sialkot Pakistan