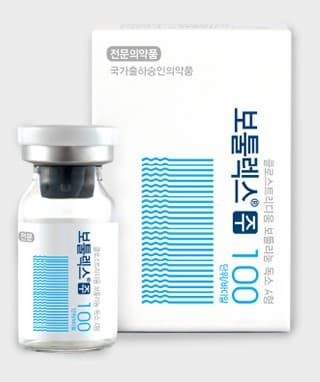
Botulax 100U
Botulinum Toxin A, Botulax
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- South Korea
- Payment Terms
- L/C,Others,T/T,Western Union
- Production method
- Available
- Shipping / Lead Time
- Negotiable / Negotiable
- Keyword
- medical product
Athena International Co., Ltd.
- Membership
- PRO
- Recent Visit
- Jan 07, 2025
- Country / Year Established
-
 South Korea
/
2022
South Korea
/
2022
- Business type
- Trading Company
- Verified Certificate
-
3


| Product name | Botulax 100U | Certification | - |
|---|---|---|---|
| Category |
Medical Devices
Medical Consumables Medical Face Mask Other Medical Consumables |
Ingredients | cbfc26,has,nacl |
| Keyword | medical product | Unit Size | - |
| Brand name | - | Unit Weigh | 30 g |
| origin | South Korea | Stock | 5000 |
| Supply type | Available | HS code | 3002 |
Product Information
BOTULAX, Botulinum Toxin A is the number one non surgical cosmetic procedure for both men and women which is produced from fermentation of Hall strain Clostridium.
It has three advantages over other similar products:
It is safe and has minimal side effects
It starts acting in just a few hours after the injection. The resulting effect is visible following 3-7 days.
BOTULAX, Botulinum Toxin A is the number one non surgical cosmetic procedure for both men and women which is produced from fermentation of Hall strain Clostridium.
A also hastherapeutic applications, Botulium Toxin Type A is used for the treatment of benign essential blepharospasm in patients 18 years of age andabove.
Botulax 100 iu contains 100 units (U) of Clostridium botulinum toxin typeA, 0.5 milligrams of albumin(human)and 0.9 milligrams of sodium chloride in a sterile, vacuum-dried from without a preservative.
Ingredient
Clostridium botulinum toxins typeA (100 units).CBFC26)
- Stabilizer: Human serumalbumin
(HAS)0.5mg
- Tonic Adjuster: Sodiumchloride (KP) 0.9mg (NACL)
Keep sealed and store at 2~8°C
A white injectable dry powder. It should become acolorless,
transparent liquid when reconstituted with a solvent. (saline solution)
36 months from the date ofmanufacture
Effectiveness
- Used for blepharospasm to adultsover 18.
-It is most often used on forehead lines, crow’s feet (lines around the eye) and frown lines.
-Temporary improver of middle of theforehead.
- The injected muscle can no longer contraction, which causes the wrinkles to relax andsoften.
B2B Trade
| Price (FOB) | Negotiable | transportation | Air Transportation,Express,Negotiation Other,Ocean Shipping |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | L/C,Others,T/T,Western Union | Shipping time | Negotiable |
- President
- Stella Chung
- Address
- #302, Shelton Building , 21, Jong-ro 44-gil, Jongno-gu, Seoul, Korea
- Product Category
- Medical Devices,Medical Test Kit,Other Medical Consumables
- Year Established
- 2022
- No. of Total Employees
- 1-50
- Company introduction
- Main Markets
-
 Japan
Japan
 Malaysia
Malaysia
 Taiwan
Taiwan
 U.A.E.
U.A.E.
 U.S.A
U.S.A
 Viet Nam
Viet Nam
- Main Product