Ranidase
Treatment Korea company is wholesale dealer from South Korea. Direct fast shipping from manufacturer. We sell most popular dermal Fillers, Botox, Lipo
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- South Korea
- Payment Terms
- Others,T/T
- Production method
- Available
- Shipping / Lead Time
- Negotiable / Negotiable
- Category
- Other Beauty Equipment
Celset Co., Ltd.
- Membership
- PRO
- Recent Visit
- Jan 23, 2025
- Country / Year Established
-
 South Korea
/
South Korea
/
- Business type
- Others
- Verified Certificate
-
3


| Product name | Ranidase | Certification | - |
|---|---|---|---|
| Category | Other Beauty Equipment | Material | - |
| Keyword | injection , pdrn | Unit Size | - |
| Brand name | - | Unit Weigh | - |
| origin | South Korea | Stock | 1000 |
| Supply type | Available | HS code | - |
Product Information
Instruction for use:
Adults, children and elderly
1. For subcutaneous injection (massive subcutaneous injection)
As for hyaluronidase, dissolve 1,500 I.U. in 1 mL of water for injection or physiological saline injection and inject into the affected area before starting subcutaneous injection, or inject into the tube 2cm above the injection needle when starting the injection. When administering 500~1,000mL of infusion, 1,500 I.U. of this product is appropriate. In children and the elderly, the rate and total dose at the time of administering fluids should be carefully controlled, especially in case of renal impairment, to avoid excess fluid.
2. For subcutaneous or intramuscular injection
Dissolve about 1,500 I.U. of this product directly in the injection to be administered.
3. Local anesthetic
Dissolve 1,500 I.U. of this product in the injection solution of the local anesthetic to be administered and used. When used in ophthalmology, a concentration of 15 I.U. per mL is recommended.
4. Extravasation
In the case of diffuse rather than local, as soon as possible after the appearance of extravasation, dissolve 1,500 I.U. of this product in 1 mL of water for injection or physiological saline solution to infiltrate the lesion site.
5. Hematoma
Dissolve 1,500 1.U. of this drug in 1mL of water for injection or physiological saline injection and infiltrate the affected area. Immediately before use, this drug is dissolved in the injection solution to be administered with about 1mL of water for injection.
The subcutaneous injection solution should be present with the extracellular fluid in the body. This drug can be combined with commonly used fluids. An example of using physiological saline injection, 0.18% sodium chloride 4% glucose, 0.45% sodium chloride 2.5% glucose, and 5% glucose injection for bulk subcutaneous injection has been reported. Potassium is administered to isotonic glucose or physiological saline at a rate of 34 mmol/L. An infusion solution containing an electrolyte is more suitble than an infusion solution containing no electrolyte, and should not be injected too quickly.
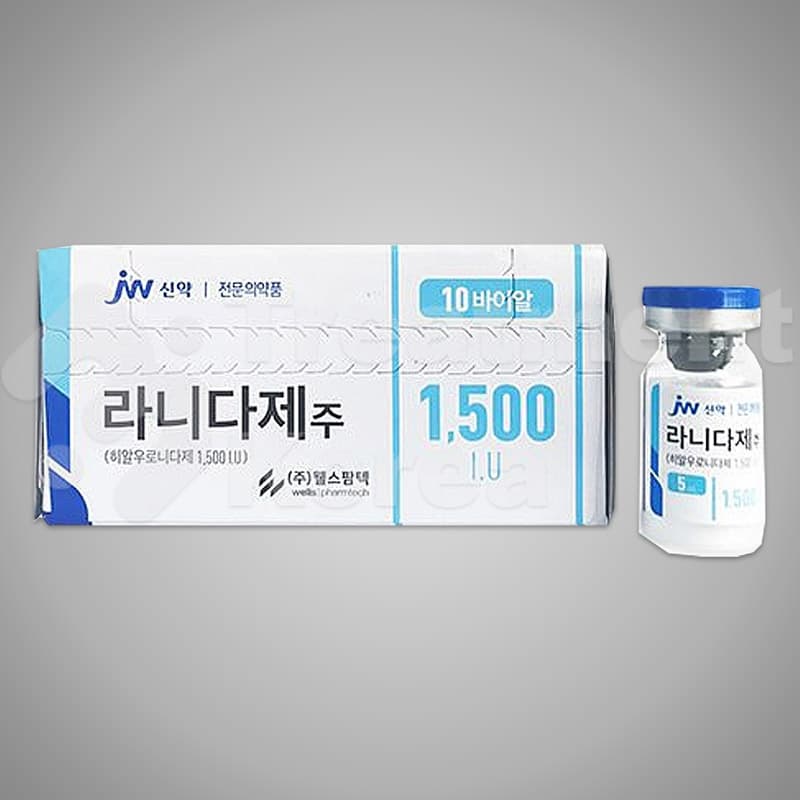
Packaging : 10 vials / box
Specification : 5ml / vial
Ingredient : 1,500 I.U. of Hyaluronidase
Shelf life : 2 years
Efficacy : For failed HA Filler operation, to dissolve HA.
Country of Origin : Republic of Korea
B2B Trade
| Price (FOB) | Negotiable | transportation | Negotiation Other |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | Others,T/T | Shipping time | Negotiable |
- President
- Product Category
- Medical Devices
- Main Markets
-
 Indonesia
Indonesia
 Philippines
Philippines
 Russia
Russia
 Thailand
Thailand
- Main Product
Related Products
HIFU(High Intensity Focused Ultrasound)
Diode Hair Removal device - OLIVE PLUS
New Otoderm LED Plus Oxygen therapy machine for special skin care

Deesse Mask, LED Mask

Hairview



































_2.jpg)