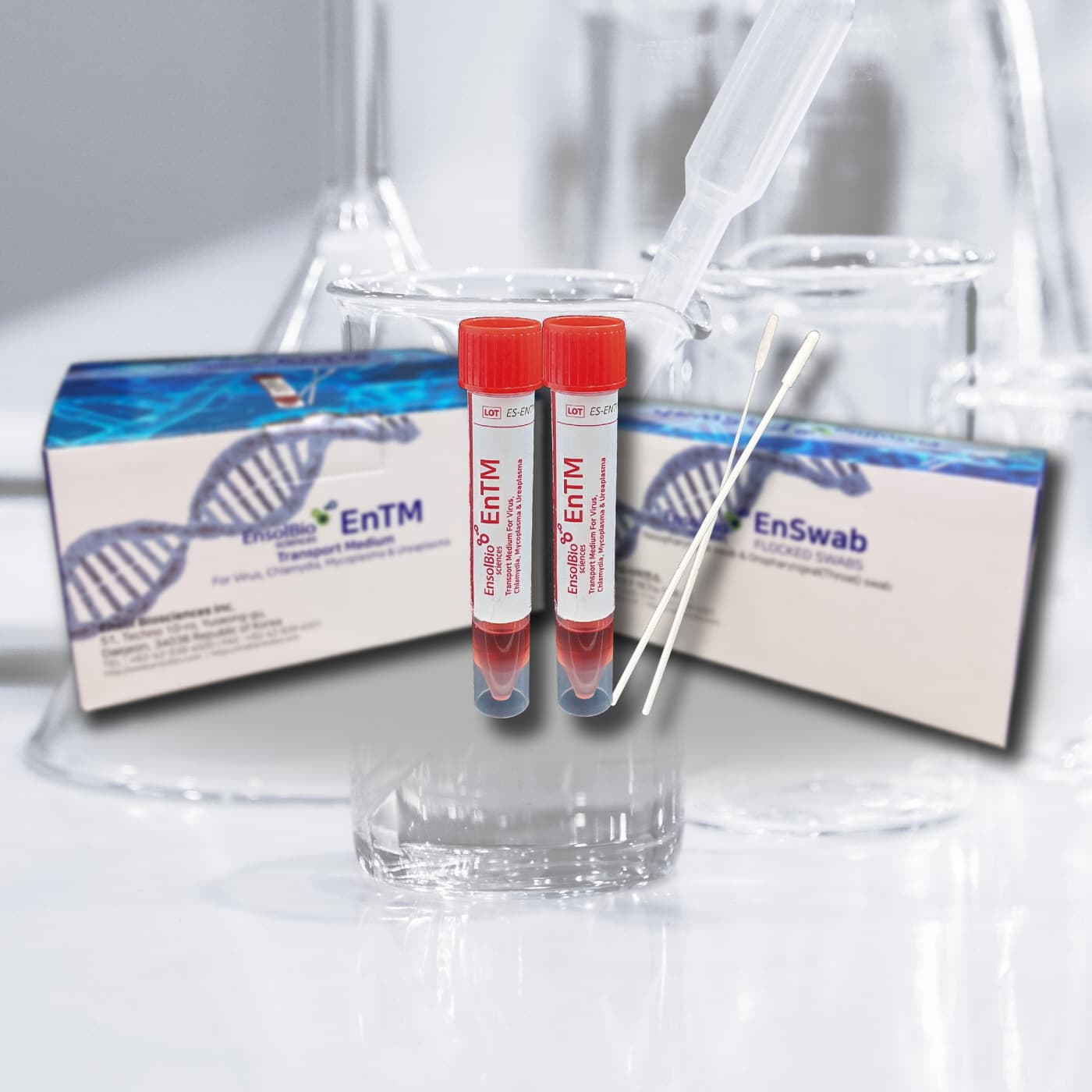
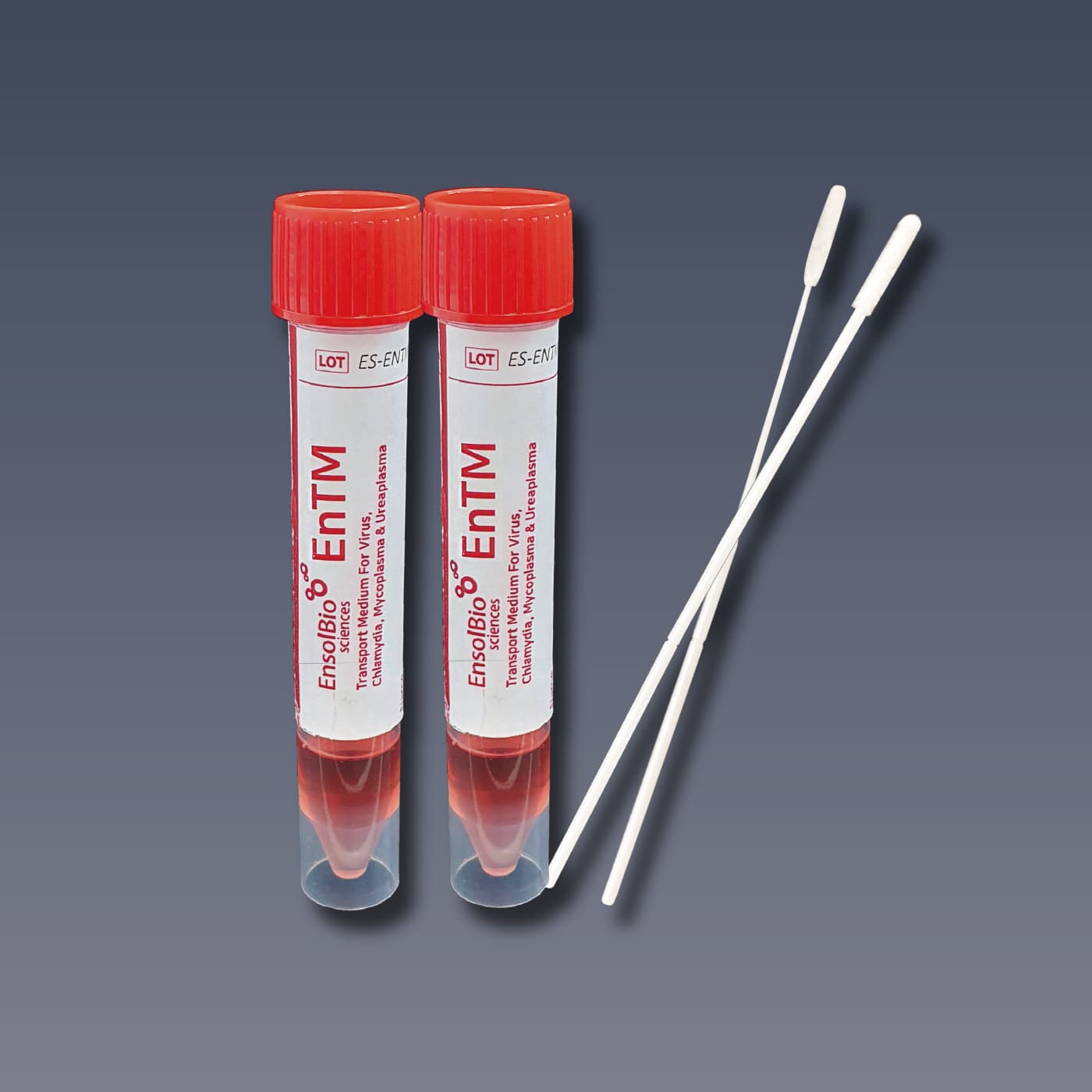
VTM, UTM, Viral Transport Media, EnTM Collection and Transport System
Viral Transport Media, Flocked Swabs with break point
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- South Korea
- Brand name
- EnTM Collection and Transport System
- Payment Terms
- T/T
- Production method
- Available
- Shipping / Lead Time
- Negotiable / Negotiable
- Keyword
- utm, swab sticks, vtm, viral transport media
| Product name | VTM, UTM, Viral Transport Media, EnTM Collection and Transport System | Certification | FDA , CE |
|---|---|---|---|
| Category |
Emergency & Clinics Apparatus
Sterilization Equipment Medical Test Kit Other Monitoring & Diagnostic Equipment |
Ingredients | ANTIBIOTICS AND ANTIMYCOTICS TO PREVENT OVERGROWTH OF BACTERIAL AND FUNGAL FLORA AND A BUFFER SOLUTION TO MAINTAIN A NEUTRAL PH.,ENSOL ENTM FORMULATION INCLUDES PROTEINS FOR VIRUS STABILIZATION |
| Keyword | utm , swab sticks , vtm , viral transport media | Unit Size | 65.5 * 49.0 * 39.5 cm |
| Brand name | EnTM Collection and Transport System | Unit Weigh | 17 kg |
| origin | South Korea | Stock | 300 |
| Supply type | Available | HS code | 3821000000 |
Product Information
INTENDED USE |
Ensol EnTM Specimen Collection and Transport System (EnTM KIT) is intended for the collection and transport of clinical samples containing viruses from the collection site to the testing laboratory. The Ensol EnTM can be used in the laboratory to perform viral culture. EnTM's medium serves as a culture media, non-propagating transport.
STORAGE |
The product must be stored in its original packaging at a temperature between 4 and 25 °C until the time of use. Do not overheat or freeze prior to use.
SHELF LIFE |
EnTM has a shelf life of 12 months when stored at 4-25°C.
INSTRUCTIONS FOR USE |
Proper collection of the specimen from the patient is a crucial aspect for successful isolation and identification of infectious organisms. Specimens should be collected as soon as possible after the clinical onset of disease. Highest viral titers are present during the acute illness.
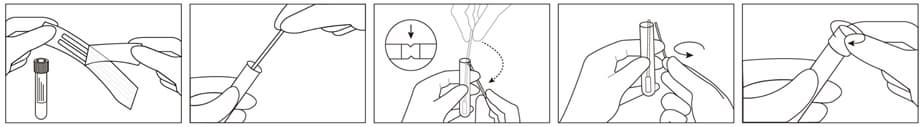
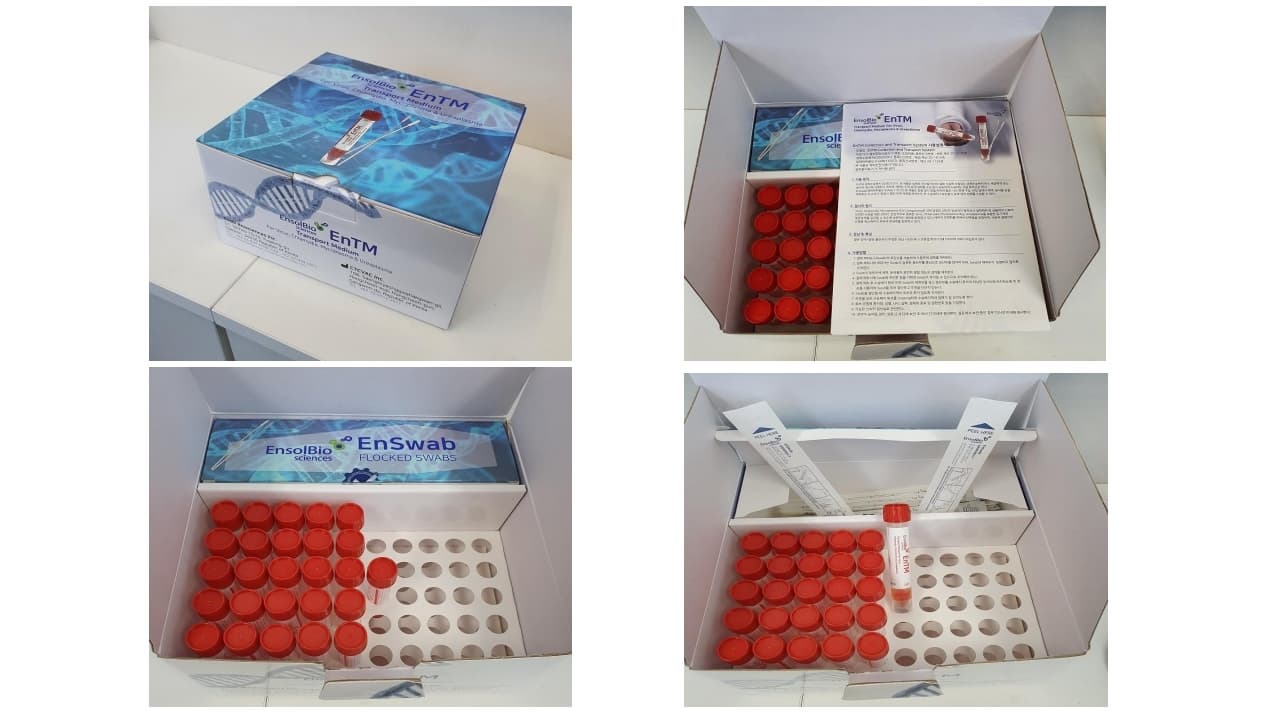
1. Peel open sterile pouch containing swabs.
2. Remove swab from the pouch and collect specimen. Ensure the applicator tip only touches the suspected infectious area to minimize potential contamination.
3. After opening the cap and put the swab specimen into the transport medium, carefully break the swab shaft against the breakpoint using hands and discard the top portion of swab without a splash.
4. Recap the transport medium tube tightly.
5. Record the name of specimen, sex, age, number, date, etc.
If processing is delayed (over 72 hours), the specimens must be frozen at -70°C or colder.
Cat.No. | VTM | SWAB | PACKAGING |
ES-TM-01 | The tube contains about 2ml of pale brown to red color medium solution. Tube size : 16 × 105 mm | NP Swab 1 EA & Throat Swab 1 EA / pc Sterilized by E-beam irradiation | 50 kits / box |
ES-TM-02 | The tube contains about 2ml of pale brown to red color medium solution. Tube size : 16 × 105 mm | - | 50 tubes / box |
- Product Info Attached File
- Verified Certificate
-


B2B Trade
| Price (FOB) | Negotiable | transportation | Air Transportation,Express |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | T/T | Shipping time | Negotiable |

- President
- Hae-Jin Kim
- Address
- 51, Techno 10-ro, Yuseong-gu, Daejeon, Korea
- Product Category
- Other Examination & Testing Instrumnet,Other Monitoring & Diagnostic Equipment,Sterilization Equipment
- Year Established
- 2001
- No. of Total Employees
- 1-50
- Company introduction
-
As a bio big data-based biopharmaceutical company that develops global innovative new drugs for diseases without therapeutic alternatives worldwide, such as degenerative disc, degenerative arthritis, TNBC, Alzheimer’s disease and type 1 diabetes, Ensol Biosciences applies bio big data-based BT/IT convergence technology from the aspects of drug efficacy, mechanism and toxicity, and side effects to develop outstanding drugs with high potential, and works on various discoveries and clinical trial phases systematically.
Ensol Biosciences power behind the development of innovative drugs that global pharmaceutical companies haven’t even solved yet and developing them into first-in-class drugs is in the ‘bio big data-based’ new drug candidate search platform, called KISDD, which the company has been developing and operating endlessly since the establishment of company in 2001.
Ensol Biosciences creates high added value through the development of innovative new drug, licensing out and commercialization, and provides CDO service and new drug CMC consigned development service based on our new drug development technology. Also, based on the synthesis, analysis and separation technique of new drug material candidates, we offer the production/sales and analysis/separation service of phytochemical, a high-value biomaterial, and synthesis of peptides and chemicals.
Main Export Product
The company expanded its business to include viral transport medium and diagnostic reagents following the COVID-19 pandemic last year. The company has developed EnTM, a viral transport medium(VTM), as a know-how in the CMC field accumulated in the process of developing new drugs. EnTM is a tool that collects and safely preserves virus and bacterial samples from human nasal and mouth for use in diagnostic testing of the viral epidemic, COVID-19.
- Main Markets
-
 Indonesia
Indonesia
 U.S.A
U.S.A
- Main Product
Related Products

I-STEM (Y-STEM) PRP KIT
AFP/PSA/CEA Rapid Test
BioTracer FOB Rapid Test

Animal Urine Analysis Test Strip Self-Stik VET
COVID 19 IgM / IgG RAPID KIT


































 South Korea
South Korea