FREND COVID19 Ag
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- South Korea
- Payment Terms
- Negotiable
- Production method
- Negotiable
- Shipping / Lead Time
- Negotiable / Negotiable
- Category
- Medical Devices
NanoEnTek Inc.
- Recent Visit
- Jan 13, 2025
- Country / Year Established
-
 South Korea
/
2000
South Korea
/
2000
- Business type
- Manufacturer
- Verified Certificate
-
17



| Product name | FREND COVID19 Ag | Certification | - |
|---|---|---|---|
| Category | Medical Devices | Ingredients | - |
| Keyword | ag , antigen , covid , covid-19 | Unit Size | - |
| Brand name | - | Unit Weigh | - |
| origin | South Korea | Stock | - |
| Supply type | - | HS code | - |
Product Information
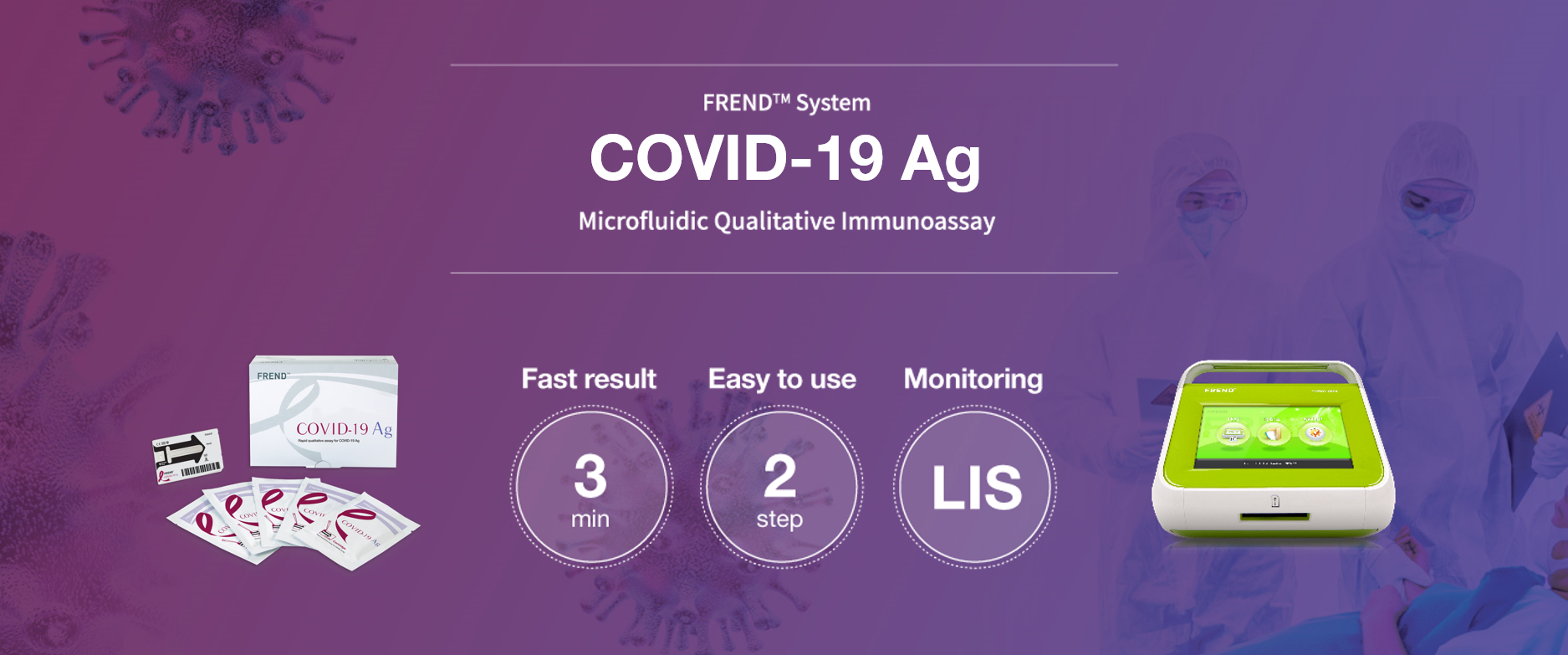
The FREND™ COVID-19 Ag is a fluorescence immunoassay (FIA) for use with the FREND™ System. It is designed for the qualitative detection of the nucleocapsid protein of SARS-CoV-2 directly from nasopharyngeal swab specimens directly from individuals suspected with COVID-19 by their healthcare provider.
FREND COVID-19 Ag is
- A fluorescence immunoassay (FIA) for use with the FREND System.
- Designed for the qualitative detection of the nucleocapsid protein of SARS-CoV-2 directly from nasopharyngeal swab specimen.
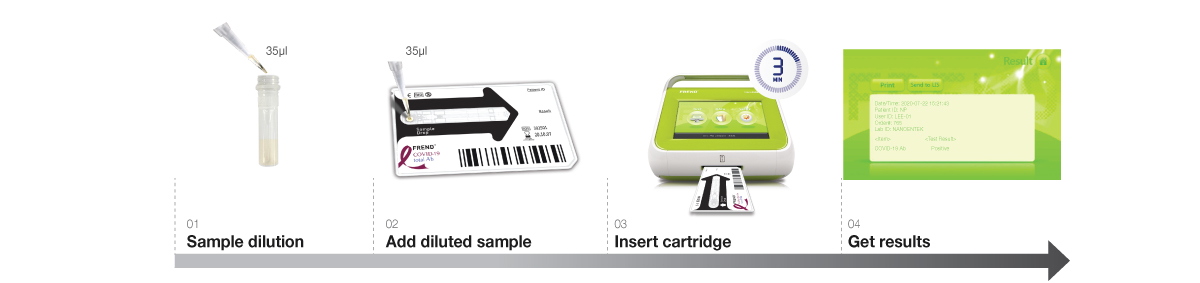
- Demonstrates reliable and objective results in just 3 minutes with one drop or 35 uL of sample.
- Cartridge designed for insertion into FREND system.
KEY FEATURES & BENEFITS
- 3 minutes | Fast result
- 2 steps | Easy to use
- 94.12% & 100% | Positive & Negative Percent Agreement
- Microfluidic Qualitative Immunoassay
- LIS connectivity (data management)
FREND COVID-19 Ag is a single use fluorescence immunoassay (FIA) kit that can detect the presence of the nucleocapsid protein of SARS-CoV-2 in nasopharyngeal swab specimen via sandwich immunoassay. The lysis buffer extracts virus from the swab specimen and releases viral proteins.
Within FREND COVID-19 Ag cartridge, the released nucleocapsid proteins are captured by antibody specific to the nucleocapsid protein from SARS-CoV-2 and detected by antibody conjugated to fluorescent micro-particles. The FREND System analyzes fluorescence intensity of control zone for validity of the test and the test zone for the presence of the nucleocapsid protein and displays the result on the screen.
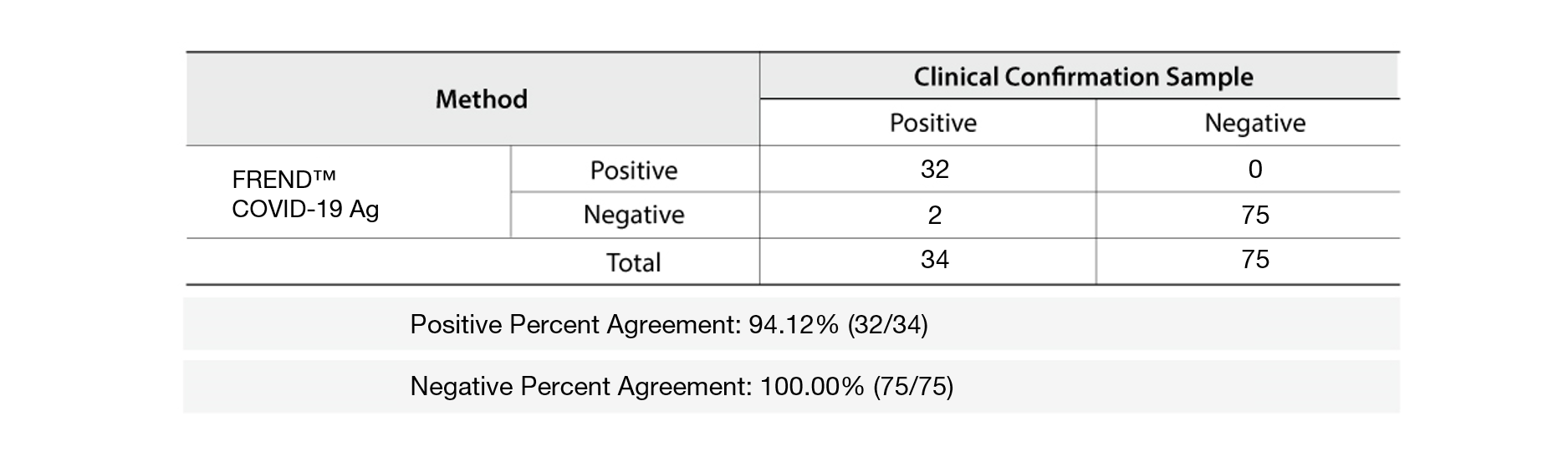
Clinical Performance Evaluation
A total of 109 clinical samples (34 positive and 75 negative) confirmed with RT-PCR were tested with the FREND™ COVID-19 Ag. It shows 94.12% PPA (Positive Percent Agreement) and 100% NPA (Negative Percent Agreement) as shown below.
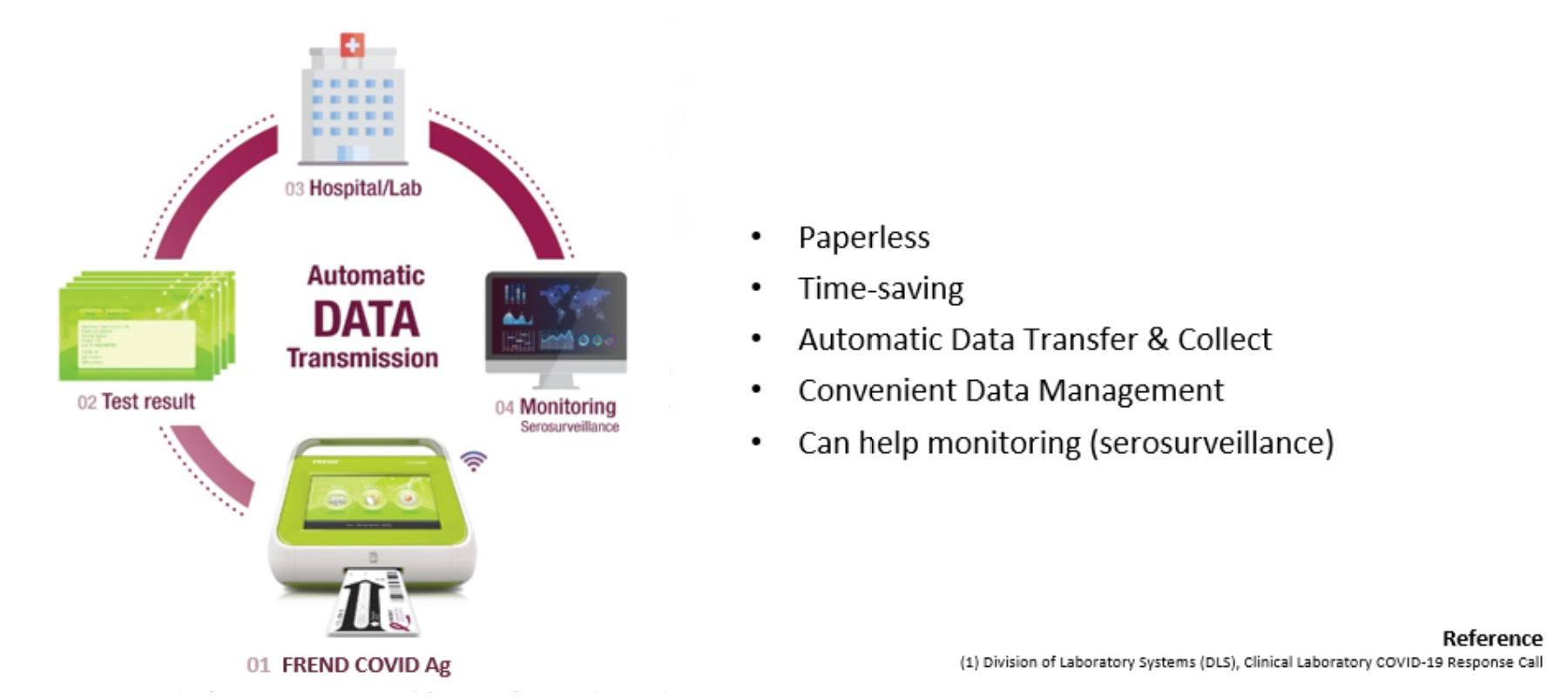
Effective Data Management
Laboratory Information System Connectivity
In the event of a pandemic, the management of a vast amount of clinical result is important. However, many laboratories face the challenge in arranging the essential information effectively(1).
The FREND™ System which is LIS compatible provide following features:
Specification
| Item | Specification |
| Assay method | Fluorescence immunoassay |
| Sample type | Nasopharyngeal swab |
| Sample volume | 35 uL |
| Time to result | < 3 min |
| Quantity | 20 tests/kit |
| Storage condition | 2 ~ 30 °C (35 ~ 86 °F) |
For more information, please visit www.nanoentek.com
B2B Trade
| Price (FOB) | Negotiable | transportation | - |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | Negotiable | Shipping time | Negotiable |
- President
- Chanil Chung
- Address
- Guro-gu, Guro-dong,235-2, Guro-gu, Seoul, Korea
- Product Category
- Medical Devices
- Year Established
- 2000
- No. of Total Employees
- 101-500
- Company introduction
-
- Main Markets
-
 Germany
Germany
 Italy
Italy
 Japan
Japan
 Taiwan
Taiwan
 U. Kingdom
U. Kingdom
- Main Product