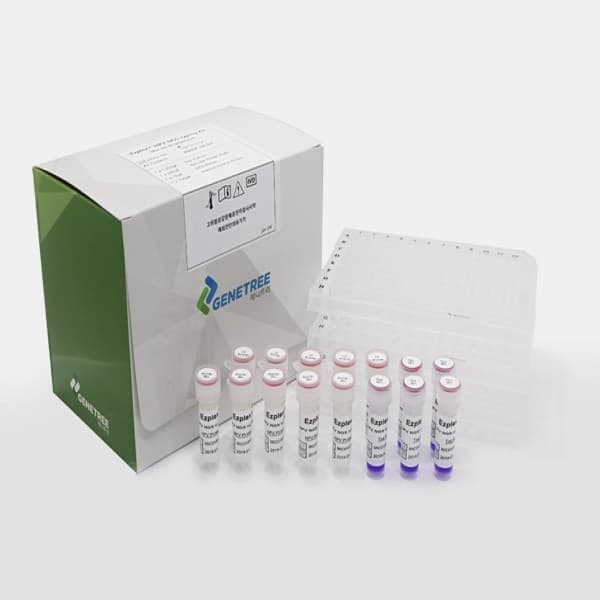
Ezplex HPV NGS typing Kit
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- South Korea
- Payment Terms
- Negotiable
- Production method
- Negotiable
- Shipping / Lead Time
- Negotiable / Negotiable
- Keyword
- kit, diagnosic kits, hpv, ivd
- Category
- Medical Test Kit
SML Genetree Co.,Ltd.
- Country / Year Established
-
 South Korea
/
2015
South Korea
/
2015
- Business type
- Manufacturer
- Verified Certificate
-
5


| Product name | Ezplex HPV NGS typing Kit | Certification | - |
|---|---|---|---|
| Category | Medical Test Kit | Ingredients | - |
| Keyword | kit , diagnosic kits , hpv , ivd | Unit Size | - |
| Brand name | - | Unit Weigh | - |
| origin | South Korea | Stock | - |
| Supply type | - | HS code | 2852105000 |
Product Information
The Ezplex® HPV NGS kit is an in vitro diagnostic medical device that detects or simultaneously detects the genotype of human papillomavirus (HPV) using Next Generation Sequencing technology (NGS). NGS instrument uses Ion Chef/S5 automated workflow to dramatically reduce hands-on time, making it easier to analyze 100 types of HPV including high risk types.
The Ezplex® HPV kit contains all reagents for library construction, such as PCR reaction mixture, primer mixture, barcode plate, and water. Also, it contains UNG(Uracil-N-glycosylase) in PCR reaction Mixture to prevent carry over contamination.
▶Product Composition
-240 Test
Product Name | Component |
| Volume | Quantity |
Ezplex® HPV NGS typing Kit | Taq Mix | Vial | 604 ㎕ | 5 |
Barcode Primer Plate | 96 well plate | 6㎕/well | 3 | |
HPV Primer Mix | Vial | 50㎕ | 5 | |
Internal control Primer | Vial | 50㎕ | 5 |
With our superior “Amplification barcoding” technology and software, we provide fast and accurate results from library construction to genotype analysis
Case No. | Assemble (Type) | #Sequence (Depth coverage) | Result |
1 | N | ≥50 | N Type Positive |
2 | N | <50 | N Type Negative |
3 | N1 | ≥50 | N Type Positive |
N2 | <50 | ||
4 | N1 | ≥50 | N1, N2 Type All Positive |
N2 | ≥50 | ||
5 |
Uncategorized |
>0 | Other than HPV gene (HPV negative) |
With our superior “Amplification barcoding” technology and software, we provide fast and accurate results from library construction to genotype analysis
HPV type analysis results can be found in software. The results of the analysis are exported to Excel and the virus type and quantity (+,++,+++) are displayed. If a co-infected HPV type is present, all types are displayed regardless of the number of infection types.
Depth Range | Positive Level |
50~500 | + |
500~5000 | ++ |
5000~ | +++ |
* +: weak risk
++: high risk
▶Clinical Sensitivity & Specificity
Clinical sensitivity and specificity were 98.1%~100% for HPV types and 100% for HPV types, respectively. As a result of comparison with other test methods, the correlation between positive samples ranged from 88.9% to 100%, and the correlation between negative samples was 99.4% to 100%
▶How to save
Constituent reagent name | Open or not | Storage conditions | Validity | Remark |
Taq Mix | Unopened | -20℃ below | 6 months from the date of manufacture | complete product |
Barcode Primer Plate | Unopened | -20℃ below | 6 months from the date of manufacture | complete product |
HPV Primer Plate | Unopened | -20℃ below | 6 months from the date of manufacture | complete product |
Internal control Primer | Unopened | -20℃ below | 6 months from the date of manufacture | complete product |
- Product Info Attached File
B2B Trade
| Price (FOB) | Negotiable | transportation | - |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | Negotiable | Shipping time | Negotiable |
SML Genetree Co.,Ltd.
- Country / Year Established
-
 South Korea
/
2015
South Korea
/
2015
- Business type
- Manufacturer
-
5


- President
- AN JI HUN
- Address
- 6F Hanmaeum Bldg 225 Bauroiro, Seocho-gu, Seoul, Korea
- Product Category
- Other Monitoring & Diagnostic Equipment
- Year Established
- 2015
- Company introduction
-
- Main Product