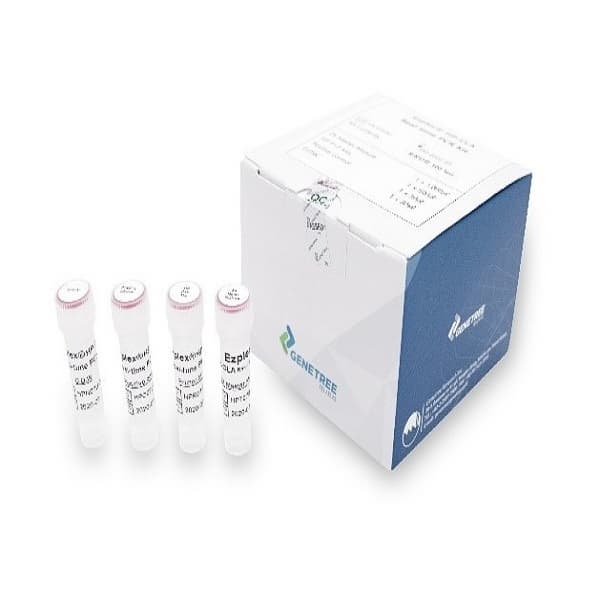
Ezplex HP-CLA Real-time PCR Kit
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- South Korea
- Payment Terms
- Negotiable
- Production method
- Negotiable
- Shipping / Lead Time
- Negotiable / Negotiable
- Keyword
- test, diagnosis reagent, ivd, pyiori
- Category
- Medical Test Kit
SML Genetree Co.,Ltd.
- Country / Year Established
-
 South Korea
/
2015
South Korea
/
2015
- Business type
- Manufacturer
- Verified Certificate
-
5


| Product name | Ezplex HP-CLA Real-time PCR Kit | Certification | - |
|---|---|---|---|
| Category | Medical Test Kit | Ingredients | - |
| Keyword | test , diagnosis reagent , ivd , pyiori | Unit Size | - |
| Brand name | - | Unit Weigh | - |
| origin | South Korea | Stock | - |
| Supply type | - | HS code | 2852105000 |
Product Information
Ezplex® HP-CLA Real-time PCR Kit is a reagent that uses the PCR method to qualitatively detect helicobacter pylori and clarismycin-resistant mutations from human driven gastric biopsy tissues. It can test 96 samples at once, quickly and easily with a short time PCR response, along with dedicated software to help you see the results.
▶Analytical Sensitivity
Repeated tests with serial dilutions of the standard material have confirmed the high analytical sensitivity of Ezplex® HP-CLA Real-time PCR Kit
Target | Limit of Detection (copies/㎕) | |
Helicobacter pylori | 3.604 | |
Clarithromycin resistance mutations | A2142G | 4.174 |
A2143G | 4.626 | |
▶Reproducibility
Reproducibility tests using standard materials all showed stable results of less than 5% CV.
Target | Density | Mean | CV (5%) | |
Helicobacter pylori | Medium concentration | 30.81 | 1.14 | |
Low concentration | 34.51 | 0.88 | ||
Clarithromycin resistance mutations |
A2142G | Medium concentration | 31.03 | 0.84 |
Low concentration | 34.39 | 0.84 | ||
A2143G | Medium concentration | 30.93 | 0.83 | |
Low concentration | 34.19 | 0.92 | ||
▶Stability
Ezplex® HP-CLA Real-time PCR Kit was tested reapeatedly at the initial time point and at each storage point for 6 months and 12 months, and both showed CV less than 5%, confirming that it maintains stability.
Target | Density | Initial mean | 6 months mean | 12 months mean | Total CV (%) | |
Helicobacter pylori | Medium
| 31.00 | 31.41 | 31.57 | 1.17 | |
Low
| 34.56 | 35.06 | 35.27 | 1.28 | ||
Clarithromycin resistance mutations |
A2142G | Medium
| 31.15 | 31.61 | 31.90 | 1.05 |
Low
| 34.47 | 34.95 | 35.37 | 1.12 | ||
A2143G | Medium
| 31.02 | 31.46 | 31.65 | 0.93 | |
Low
| 34.02 | 34.58 | 34.90 | 1.14 | ||
▶Clinical Accuracy
In a test conducted by the general clinical laboratories in Korea using human stomach biopsy samples that were already confirmed by the KFDA(Korea Food and Drug Ministration), our high clinical performance was validated.
Helicobacter pylori | Control group (licensed product) | ||
Positivity | Negativity | ||
Ezplex®HP-CLA Real-time PCR Kit | Positivity | 82 | 0 |
Negativity | 0 | 66 | |
Clinical sensitivity (95%CI) | 100% (95.60~100%) | ||
Clinical specificity (95%CI) | 100% (94.56~100%) | ||
A2142G | Control group (licensed product) | ||
Positivity | Negativity | ||
Ezplex®HP-CLA Real-time PCR Kit | Positivity | 47 | 0 |
Negativity | 0 | 66 | |
Clinical sensitivity (95%CI) | 100% (92.45~100%) | ||
Clinical specificity (95%CI) | 100% (94.56~100%) | ||
A2143G | Control group (licensed product) | ||
Positivity | Negativity | ||
Ezplex®HP-CLA Real-time PCR Kit | Positivity | 70 | 0 |
Negativity | 0 | 66 | |
Clinical sensitivity (95%CI) | 100% (94.87~100%) | ||
Clinical specificity (95%CI) | 100% (94.56~100%) | ||
▶Ordering information
Ezplex®HP-CLA Real-time PCR Kit includes Taq Polymerase, Primer&Probe, Positive control and Internal control.
Product Classification | Product size | Purpose of use |
Ezplex®HP-CLA Real-time PCR Kit | 100 reaction | IVD, RUO |
Ezplex®HP-CLA Real-time PCR Kit | 200 reaction | IVD, RUO |
- Product Info Attached File
B2B Trade
| Price (FOB) | Negotiable | transportation | - |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | Negotiable | Shipping time | Negotiable |
SML Genetree Co.,Ltd.
- Country / Year Established
-
 South Korea
/
2015
South Korea
/
2015
- Business type
- Manufacturer
-
5


- President
- AN JI HUN
- Address
- 6F Hanmaeum Bldg 225 Bauroiro, Seocho-gu, Seoul, Korea
- Product Category
- Other Monitoring & Diagnostic Equipment
- Year Established
- 2015
- Company introduction
-
- Main Product