Ezplex SARS-CoV-2 G Kit (FAST)
Medical equipment reagent
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- South Korea
- Payment Terms
- T/T
- Production method
- Available
- Shipping / Lead Time
- Negotiable / Negotiable
- Keyword
- fast, real-time, covid, molecular diagnosis
SML Genetree Co.,Ltd.
- Country / Year Established
-
 South Korea
/
2015
South Korea
/
2015
- Business type
- Manufacturer
- Verified Certificate
-
4


| Product name | Ezplex SARS-CoV-2 G Kit (FAST) | Certification | CE |
|---|---|---|---|
| Category | Other Monitoring & Diagnostic Equipment | Ingredients | - |
| Keyword | fast , real-time , covid , molecular diagnosis | Unit Size | - |
| Brand name | - | Unit Weigh | - |
| origin | South Korea | Stock | 500000 |
| Supply type | Available | HS code | 382200 |
Product Information
The Ezplex® SARS-CoV-2 G Kit (FAST) is a real-time reverse transcription polymerase chain reaction (rRT-PCR) test.The 2019-nCoV primer and probe set(s) is designed to detect RNA from the SARS-CoV-2 Virus in Upper Respiratory(Nasopharyngeal/Oropharyngeal) swabs and Lower Respiratory specimens(Sputum) from patients with signs and symptoms of infection who are suspected of COVID-19.
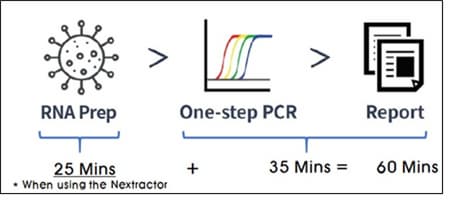
Workflow
Compatible Device
CFX96 Real-time PCR Instrument
QuantStudio 6 Flex Real-time PCR Instrument
Performance Evaluation
1) Analytical Sensitivity
Using standard SARS-CoV-2 material, it was serially spiked in both of Upper respiratory(Nasopharyngeal) and Lower respiratory specimens(Sputum), and RNA were extracted from those prepared specimens. The test was performed 20 times on every diluted concentrations and the limit of detection is calculated as below using probit analysis of 95% positive rate.
Target | CFX96 | QuantStudio 6 Flex | ||
Upper | Lower | Upper | Lower | |
RdRp | 1.585 copies/uL | 1.367 copies/uL | 2.587 copies/uL | 2.587 copies/uL |
N | 1.045 copies/uL | 1.259 copies/uL | 2.272 copies/uL | 1.887 copies/uL |
2) Analytical Specificity
2.1) Cross reactivity
The 15 species of microorganisms, which are expected of cross reactivity, are chosen for the test and the test repeated three times on every chosen species. As a result of the test, there were no cross reactivity as those were observed as all negative result.
Microorganism | SARS- CoV-2 (O) | SARS-CoV-2 (X) | Microorganism | SARS- CoV-2 (O) | SARS- CoV-2 (X) |
Influenza A H3 | Pos | Neg | Coronavirus 229E | Pos | Neg |
Influenza B | Pos | Neg | Enterovirus 71 | Pos | Neg |
Bordetella pertussis | Pos | Neg | Adenovirus | Pos | Neg |
Respiratory Synncytial virus B | Pos | Neg | Rhinovirus | Pos | Neg |
Parainfluenza virus 1-3 | Pos | Neg | Bordetella parapertussis | Pos | Neg |
Coronavirus OC43 | Pos | Neg | Legionella pnermophila | Pos | Neg |
Coronavirus NL63 | Pos | Neg |
| ||
2.2) Interference
The interference materials were prepared with 3 materials of endogenous - Albumin (0.24g/mL), Hemoglobin (0.2g/mL), Bilirubin (0.05mg/mL) - those were tested three times with and without positive materials of SARS-CoV-2 diluted in low concentration. As a result of the test, there were no interference by observing the coefficient of variation(CV) value which were less than 5% in all cases.
Controls | Interference | |||||
Albumin | Hemoglobin | Billirubin | ||||
O | X | O | X | O | X | |
SARS-CoV-2 Virus | Positive (CV 5%>) | Positive | Positive (CV 5%>) | Positive | Positive (CV 5%>) | Positive |
Negative control | Negative | Negative | Negative | Negative | Negative | Negative |
3) Analytical Precision
3.1) Analytical Precision (Reproducibility)
A reference material for SARS-CoV-2 Virus(Vircell, MBC137-R) was diluted into middle concentration (100 copies/uL) and low concentration (5 copies/uL). It was tested together with negativity (D.W) using 1 Lot by 2 investigators and each investigator tested several times repeatedly. The detection results were same across the tests with CV < 5%, and it was confirmed that results did not differ between investigators.
3.2) Analytical Precision (Repeatability)
A reference material for SARS-CoV-2 Virus(Vircell, MBC137-R) was diluted into middle concentration (100 copies/uL) and low concentration (5 copies/uL). It was tested together with negativity (D.W) using 2 Lots by 1 investigator and the investigator tested 10 times repeatedly, 5 times a day for 2 days. All the detection results were same with CV < 5%, and it was confirmed that results did not differ within a test, between tests and between lots.
4)Clinical Evaluation
The clinical performance was evaluated by a clinical performance testing institution in Republic of Korea using the left-over upper respiratory (nasopharyngeal and oropharyngeal) and lower respiratory (sputum) specimens that were positive and negative through the emergency use approved product (Kosen Biotech, PowerchekTM 2019-nCoV Real-time PCR kit) by Korea CDC, and the results are as follows.
(1) CFX96 Real-time PCR Instrument
Upper (Nasopharyngeal/ Oropharyngeal) | Reference | Lower (Sputum( | Reference | ||||||
Positive | Negative | Total | Positive | Negative | Total | ||||
Ezplex® | Positive | 99 | 0 | 100 | Ezplex® | Positive | 99 | 0 | 100 |
Negative | 1 | 100 | 100 | Negative | 1 | 100 | 100 | ||
Total | 100 | 100 | 200 | Total | 100 | 100 | 200 | ||
Sensitivity (95% CI) | 99 % (94.55 ~ 99.97 %) | Sensitivity (95% CI) | 99 % (94.55 ~ 99.97 %) | ||||||
Specificity (95% CI) | 100 % (96.38 ~ 100 %) | Specificity (95% CI) | 100 % (96.38 ~ 100 %) | ||||||
(2) QuantStudio 6 Flex Real-time PCR Instument
Upper (Nasopharyngeal/ Oropharyngeal) | Reference | Lower (Sputum( | Reference | ||||||
Positive | Negative | Total | Positive | Negative | Total | ||||
Ezplex® | Positive | 30 | 0 | 30 | Ezplex® | Positive | 30 | 0 | 30 |
Negative | 0 | 30 | 30 | Negative | 0 | 30 | 30 | ||
Total | 30 | 30 | 60 | Total | 30 | 30 | 60 | ||
Sensitivity (95% CI) | 100 % (88.43 ~ 100 %) | Sensitivity (95% CI) | 100 % (88.43 ~ 100 %) | ||||||
Specificity (95% CI) | 100 % (88.43 ~ 100 %) | Specificity (95% CI) | 100 % (88.43 ~ 100 %) | ||||||
Kit Components
* 50 tests
No. | Kit Component | Volume | Lid Remarks |
1 | RQ Mixture | 1 vial, 600uL | RQ |
2 | RdRp P+P | 1 vial, 200uL | RdRp |
3 | N P+P | 1 vial, 200uL | N |
4 | Positive Control | 1 vial, 100uL | PC |
5 | Negative Control | 1 vial, 100uL | NC |
6 | Internal Control | 1 vial, 20uL | IC |
* 100 tests
No. | Kit Component | Volume | Lid Remarks |
1 | RQ Mixture | 2 vials, 600uL | RQ |
2 | RdRp P+P | 2 vials, 200uL | RdRp |
3 | N P+P | 2 vials, 200uL | N |
4 | Positive Control | 1 vial, 100uL | PC |
5 | Negative Control | 1 vial, 100uL | NC |
6 | Internal Control | 1 vial, 20uL | IC |
- Verified Certificate
-

B2B Trade
| Price (FOB) | Negotiable | transportation | Air Transportation |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | T/T | Shipping time | Negotiable |
SML Genetree Co.,Ltd.
- Country / Year Established
-
 South Korea
/
2015
South Korea
/
2015
- Business type
- Manufacturer
-
4


- President
- AN JI HUN
- Address
- 6F Hanmaeum Bldg 225 Bauroiro, Seocho-gu, Seoul, Korea
- Product Category
- Other Monitoring & Diagnostic Equipment
- Year Established
- 2015
- Company introduction
-
- Main Product