BIOCREDIT COVID-19 IgG+IgM Duo
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- South Korea
- Brand name
- BIOCREDIT COVID-19 IgG+IgM Duo
- Payment Terms
- T/T
- Production method
- Negotiable
- Shipping / Lead Time
- Negotiable / Negotiable
- Keyword
- corona treater, corona virus, covid-19, covid-19 rapid test
- Category
- Medical Test Kit

Moida
- Verified Certificate
-
4


| Product name | BIOCREDIT COVID-19 IgG+IgM Duo | Certification | CE |
|---|---|---|---|
| Category | Medical Test Kit | Ingredients | - |
| Keyword | corona treater , corona virus , covid-19 , covid-19 rapid test | Unit Size | - |
| Brand name | BIOCREDIT COVID-19 IgG+IgM Duo | Unit Weigh | 10 g |
| origin | South Korea | Stock | - |
| Supply type | - | HS code | 382200 |
Product Information
RapiGEN Rapid Test
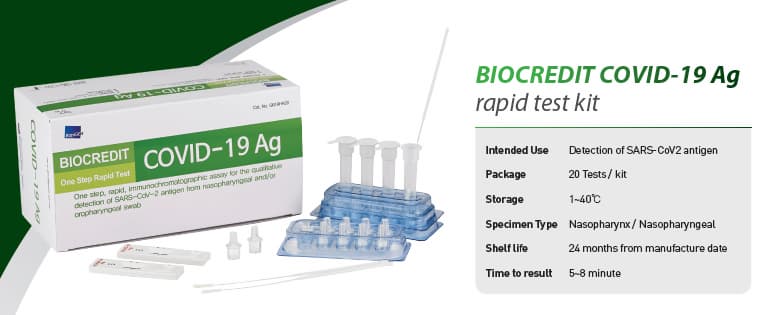
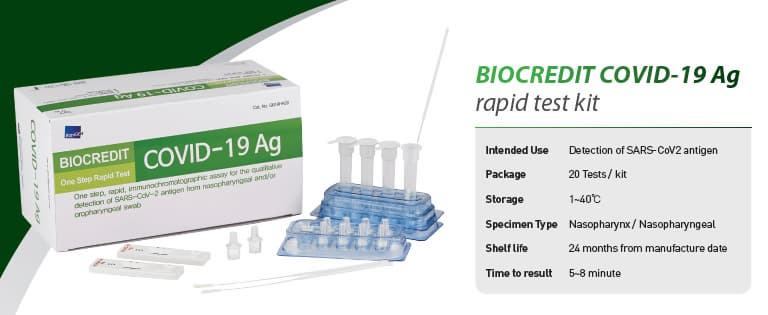
BIOCREDIT COVID-19 Ag
RAP-01-10
BIOCREDIT COVID-19 IgG+IgM Duo
RAP-01-11
█ Introduction
2019 novel coronavirus (2019-nCoV) is a single-stranded RNA
coronavirus. Coronavirus disease 2019 (COVID-19) is a respiratory
illness caused by the 2019-nCoV. 2019-nCoV belongs to the Betacoronavirus
Genus, which also includes Severe Acute Respiratory
Syndrome coronavirus (SARS-CoV, 2003) and Middle East Respiratory
Syndrome coronavirus (MERS-CoV, 2012). Coronaviruses, 2019-nCoV
consist of a four viral proteins named spike (S), envelope (E), membrane
(M), and nucleocapsid (N).
Common signs of infection include respiratory symptoms, fever, cough,
shortness of breath and breathing difficulties. In more severe cases,
infection can cause pneumonia, severe acute respiratory syndrome,
kidney failure and even death.
Standard recommendations to prevent infection spread include regular
hand washing, covering mouth and nose when coughing and sneezing,
thoroughly cooking meat and eggs. Avoid close contact with anyone
showing symptoms of respiratory illness such as coughing and sneezing.
[Principle of Test]
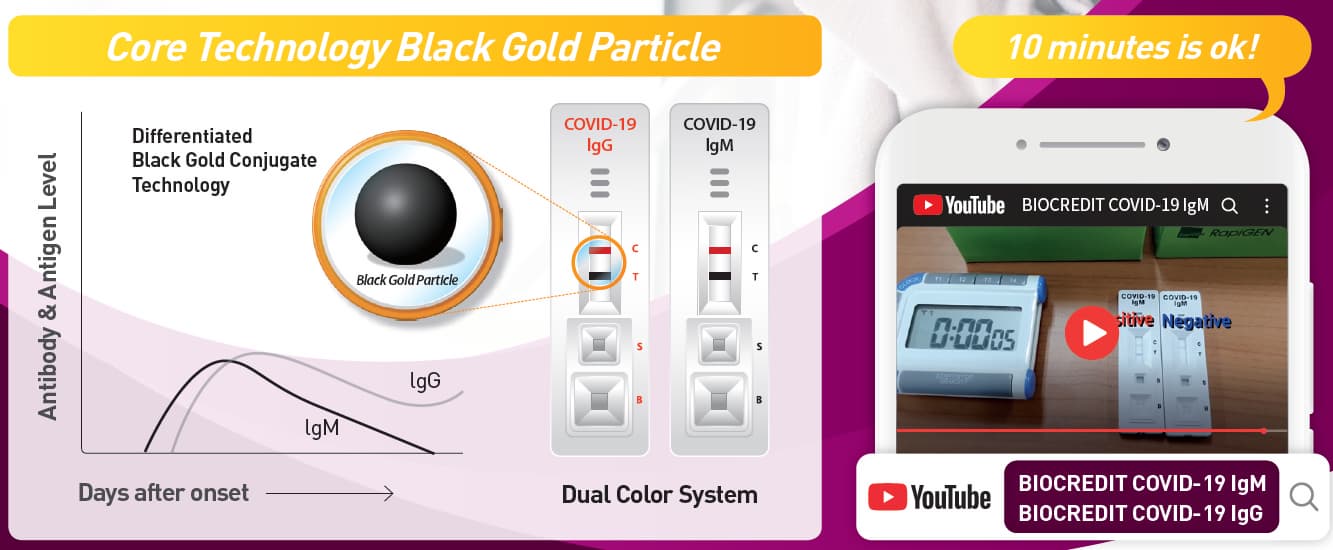
BIOCREDIT COVID-19 Ag is a lateral flow immunochromatographic
assay that adopted dual color system. The test contains colloid gold
conjugate pad and a membrane strip pre-coated with antibodies specific
to SARS-CoV-2 antigen on the test lines (T). If SARS-CoV-2 antigen is
present in the specimen, a visible black band appears on the test lines (T)
as antibody-antigen-antibody gold conjugate complex forms. The control
line (C) is used for procedural control and should always appear if the
test is performed correctly.
[Intended Use]
For the qualitative detection of SARS-CoV-2 antigen from
nasopharyngeal swab specimen
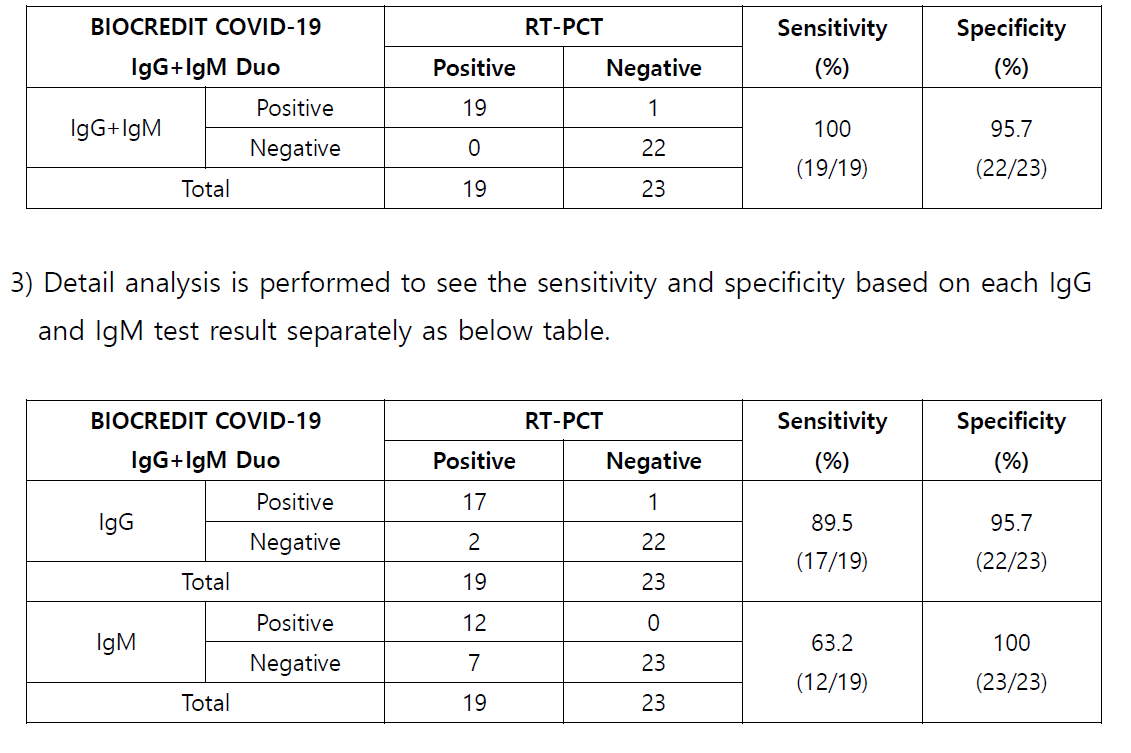
3 Conclusion
1) 19 positive specimens which were confirmed by RT PCR are tested with BIOCREDIT
COVID 19 IgG+IgM Duo kit , and all the specimens are identified as positive as well.
Relative Sensitivity (%) = 100 x (No.
of s pecimens with positive results /
No. of positive
specimen tested by RT PCR)
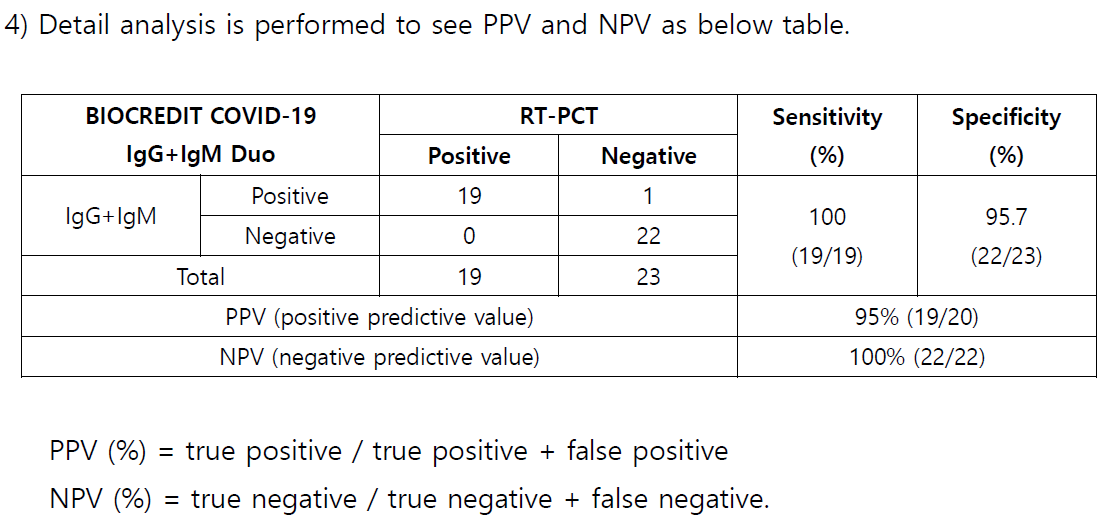
2)
23 negative specimens collected by trial center in 2019 are tested with BIOCREDIT
COVID
19 IgG+IgM Duo kit, and all the specimens except 1 (weak positive) are
identified as negative.
Relative Specificity (%) = 100 x (
No. of s pecimens with negative results /
No. of negative
s pecimens
█ Introduction
2019 novel coronavirus (2019-nCoV) is a single-stranded RNA
coronavirus. Coronavirus disease 2019 (COVID-19) is a respiratory
illness caused by the 2019-nCoV. 2019-nCoV belongs to the Betacoronavirus
Genus, which also includes Severe Acute Respiratory
Syndrome coronavirus (SARS-CoV, 2003) and Middle East Respiratory
Syndrome coronavirus (MERS-CoV, 2012). Coronaviruses, 2019-nCoV
consist of a four viral proteins named spike (S), envelope (E), membrane
(M), and nucleocapsid (N).
Common signs of infection include respiratory symptoms, fever, cough,
shortness of breath and breathing difficulties. In more severe cases,
infection can cause pneumonia, severe acute respiratory syndrome,
kidney failure and even death.
Standard recommendations to prevent infection spread include regular
hand washing, covering mouth and nose when coughing and sneezing,
thoroughly cooking meat and eggs. Avoid close contact with anyone
showing symptoms of respiratory illness such as coughing and sneezing.
[Principle of Test]
BIOCREDIT COVID-19 Ag is a lateral flow immunochromatographic
assay that adopted dual color system. The test contains colloid gold
conjugate pad and a membrane strip pre-coated with antibodies specific
to SARS-CoV-2 antigen on the test lines (T). If SARS-CoV-2 antigen is
present in the specimen, a visible black band appears on the test lines (T)
as antibody-antigen-antibody gold conjugate complex forms. The control
line (C) is used for procedural control and should always appear if the
test is performed correctly.
[Intended Use]
For the qualitative detection of SARS-CoV-2 antigen from
nasopharyngeal swab specimen
█ Kit Components
- Each test device sealed in a foil pouch with a desiccant
- Assay diluent tube
- Filter cap
- Sterilized swab for nasopharyngeal specimen collection
- Instructions for use
█ Specimen Collection and Storage
1. Specimen should be handled carefully as an infectious agent and
should be collected by trained personnel.
2. As improper collection of the sample affects the test result significantly,
handle with care.
3. More accurate results can be obtained if samples are collected from
several parts.
4. Specimen should be tested as soon as possible upon collection. If the
sample has to be stored, store the swab sample at 2~8℃ up to 12
hours or at -20℃ or below up to 24 hours.
[Nasopharyngeal swab specimen]
To collect nasopharyngeal swab specimen, gently insert a
nasopharyngeal swab into the nasal cavity until the resistance is met at
the level of the turbinate. Rotate softly and withdraw the swab. Make
sure the tip of the swab is wet.
█ Assay Procedure
[PREPARATION]
1. Equilibrate kit components and specimen to room temperature before
testing.
2. Do not break the seal of the foil pouch until ready to perform the test.
[TESTING]
1. Remove the aluminum seal of the assay diluent tube. Immerse both
nasopharyngeal swab in the assay diluent and swirl the swabs 5~10
times while pressing the head against the bottom and side of the
collection tube.
2. Withdraw the swab while pinching and squeezing. Dispose it with
biosafety.
3. Close the assay diluent tube with a filter cap securely.
4. Remove the device from the foil pouch and place it on a flat and dry
surface.
5. Invert the assay diluent tube and gently squeeze it to draw 3~4 drops
(90~150㎕) into a sample well(S) of the device.
* Please ensure that an appropriate amount of specimen and assay
diluent are used for testing. Too much or too little amount of
specimen and/or assay diluent may lead to deviation of results.
6. Read the result between 5~8 minutes.
☞Do not interpret the result after 8 minutes.
█ Interpretation of Results
[Negative]
The presence of only one red band at the control line (C) within the
result window indicates a negative result.
[Positive]
Two bands appear; one red control line(C) and one black test line(T).
[Invalid]
If the control line fails to appear within the result window, the result is
considered invalid. The directions may not have been followed correctly
or the test may have deteriorated. It is recommended that the specimen
be retested.
Note: There is no meaning attributed to line color intensity or width.
█ Performance Characteristics
BIOCREDIT COVID-19 Ag has been evaluated with panel specimen by
PCR. The results are summarized in the following table:
1. Sensitivity and Specificity:
PCR
(after symptoms
occur)
Total Sensitivity Specificity
Positive Negative
BIOCREDIT
COVID-19 Ag
Positive 23 1 24
Negative 2 49 51 92.0% 98.0%
Total 25 50 75
2. Precision
Within-run and between run precision has been determined in
triplicates of three lots using the following specimen panel: negative,
low positive, medium positive and strong positive. All specimens are
correctly identified 100% of the time.
3. Cross reactivity
BIOCREDIT COVID-19 Ag has been tested with 20 potentially cross
reacting microorganisms and viruses. The results showed that
BIOCREDIT COVID-19 Ag had no cross-reaction with microorganisms
and viruses except very weak cross reacting with SARS-coronavirus.
4. Interference
BIOCREDIT COVID-19 Ag has been tested with 14 potentially
interfering endogenous or exogenous substances. The results showed
that BIOCREDIT COVID-19 Ag had no interference with endogenous
or exogenous substances except very weak interference with HAMA
Type I.
█ Limitations
1. A negative result can occur if the quantity of coronavirus present in the
specimen is below the detection limits of the assay, or the antibodies
that are detected are not present during the stage of disease in which
a sample is collected.
2. A negative test result cannot exclude a recent infection.
█ Precautions
1. For in vitro diagnostic use only.
2. The test device is sensitive to humidity as well as heat. Perform the
test immediately after removing the test device from the foil pouch.
3. Do not use the test kit if the pouch is damaged or the seal is broken.
4. Decontaminate and dispose of all specimens, reaction kit and
potentially contaminated materials, as if they were infectious waste, in
a biohazard container.
5. Wear protective clothing, gloves and eye protection while handling
specimens. Wash hands afterwards.
6. Repeated freeze-thawing specimen can cause false positive or false
negative results.
7. Discard the solid waste by autoclaving at 121℃ for 1 hour.
8. The assay diluent contains less than 0.1% of sodium azide. In case of
dermal or eye exposure, wash out thoroughly with running water and
seek medical attention if necessary.
9. Decontaminate and dispose of all specimens, test device and
potentially contaminated materials as if they were infectious waste in a
biohazard container with biosafety.
10. Do not use it beyond the expiration date.
11. Do not reuse.
12. Do not interchange or mix reagents of different lots.
13. Other clinically available tests are required if questionable results are
obtained. As with all other diagnostic test, a clinical decision should not
be based on the results of this test, but should be made by physician
after all clinical and laboratory findings have been evaluated.
█ Package
Refer to the outer packaging
█ Storage and Shelf life
Store at 1~40℃. Shelf life is 12 months from the date of manufacture.
- Product Info Attached File
- Verified Certificate
-

B2B Trade
| Price (FOB) | Negotiable | transportation | - |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | T/T | Shipping time | Negotiable |

- President
- SEO JONG BOK
- Address
- 322 Byeollae 3-ro Namyangju-si Gyeonggi, Namyangju-si, Gyeonggi-do, Korea
- Product Category
- Face Mask,Other Facial Care,Other Health Care Products,Toothbrush Sanitizer
- Year Established
- 2016
- No. of Total Employees
- 1-50
- Company introduction
-
ㅇ 세계적으로 K방역의 관심 고조로 K방역용품의 문의 쇄도.
- 상반기 무역협회를 통해 문의만도 약 20여건으로 상반기 샘플전달을 통한 제품 신뢰도 증빙
- 하반기 수출주문 기대 하고 있습니다.





○ 동남아시아, 동남아 바이어 네트워크
바이어 초청 및 상담을 통해 수출 기반 확보

○ 중국 저장성 박람회 참석

○ 피부관리 솔루션
- 식물성원료와 단백질을 함유한 제형의 제품으로 친환경 원료를 사용한 프리미엄 토탈 관리 제품
- 수분과 영양공급을 동시에 할 수 있으며 유효성분을 피부 피부속까지 전달하고 수분 유지력을 높여줌


- Main Markets
-
 Indonesia
Indonesia
 Panama
Panama
 U.S.A
U.S.A
- Main Product
Related Products

Nurugo CPR manikin
AFP/PSA/CEA Rapid Test

PRO PRP KIT
Medium_for_collecting_corona_2.jpg)
Transport Medium(UTM)(COVID-19 sample collection KIT)
VTM, UTM, Viral Transport Media, EnTM Collection and Transport System


































 South Korea
South Korea