EXTRA HYALURONIA ACID FILLER
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- South Korea
- Brand name
- EXTRA
- Payment Terms
- T/T
- Production method
- Negotiable
- Shipping / Lead Time
- Negotiable / Negotiable
- Keyword
- filler, ha filler, tsc, crosslinking
KL GLOBAL
- Membership
- VIP
- Recent Visit
- Jan 22, 2025
- Country / Year Established
-
 South Korea
/
2005
South Korea
/
2005
- Business type
- Manufacturer
- Verified Certificate
-
11


| Product name | EXTRA HYALURONIA ACID FILLER | Certification | CE |
|---|---|---|---|
| Category |
Emergency & Clinics Apparatus
Other Medical Consumables |
Ingredients | - |
| Keyword | filler , ha filler , tsc , crosslinking | Unit Size | - |
| Brand name | EXTRA | Unit Weigh | - |
| origin | South Korea | Stock | - |
| Supply type | - | HS code | 3304999000 |
Product Information
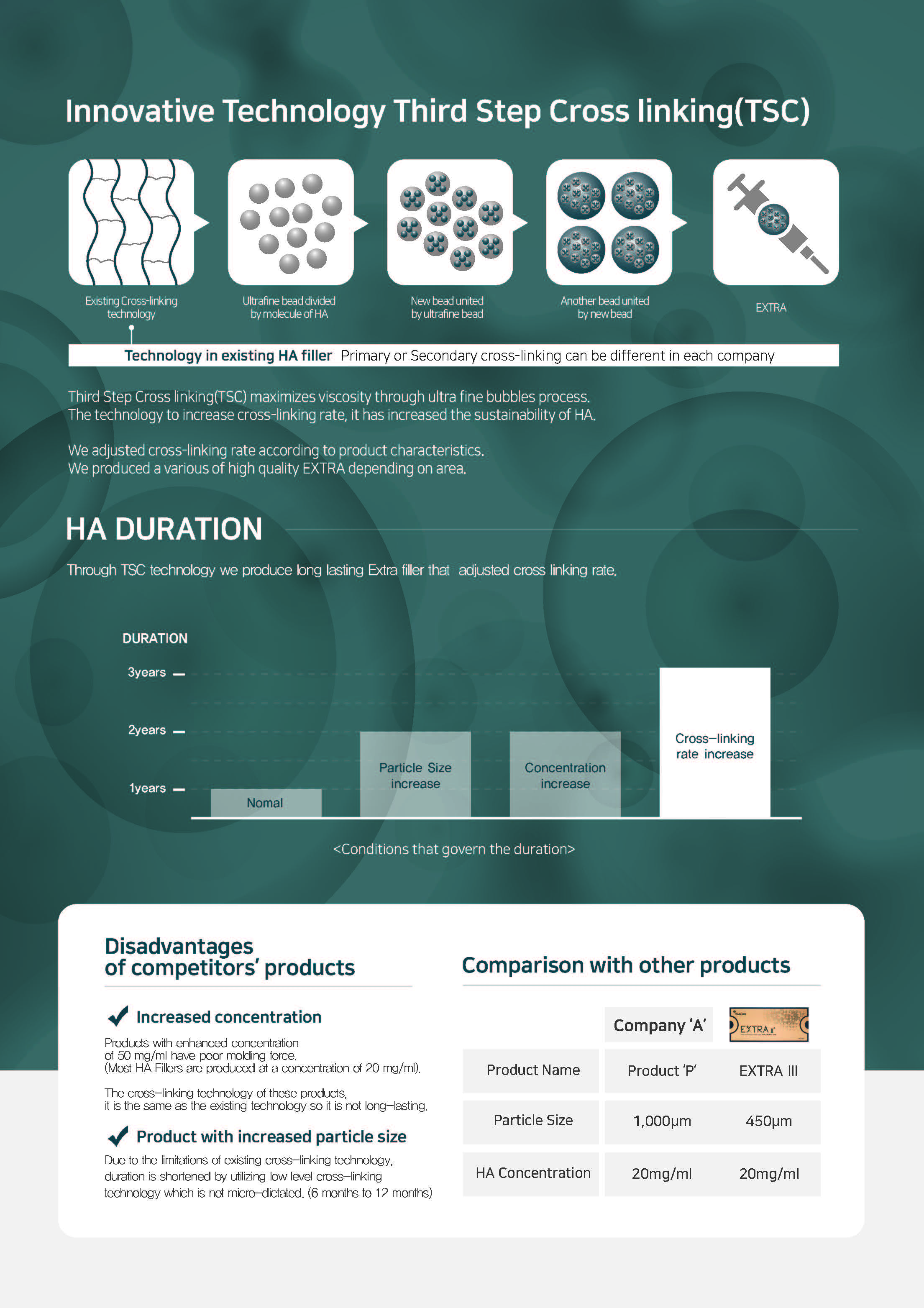
Innovative Technology
Third Step Cross linking(TSC)
Third Step Cross linking(TSC) maximizes viscosity through a patented 3step microbeads process.
The technology to increase cross-linking rate, it has increased the sustainability of HA.
We adjusted cross-linking rate according to product characteristics. We produced a various of high quality EXTRA depending on area.
DVS (Divinyl Sulfone)
cross-linked HA
EXTRA has developed MDAWO technology (Multiple Degree Amphiphilic Wash-Out technology) and applied it to processes.
The process of technology, the DVS component is completely removed and none of un-cross linked DVS is detected.
In general, the crosslinking process is carried out using DVS having a purity of about 95% for BDDE used for crosslinking HA and 97% or more purity for EXTRA. By using highly pure crosslinking bridge, crosslinking ratio is high, and high quality products can be produced.
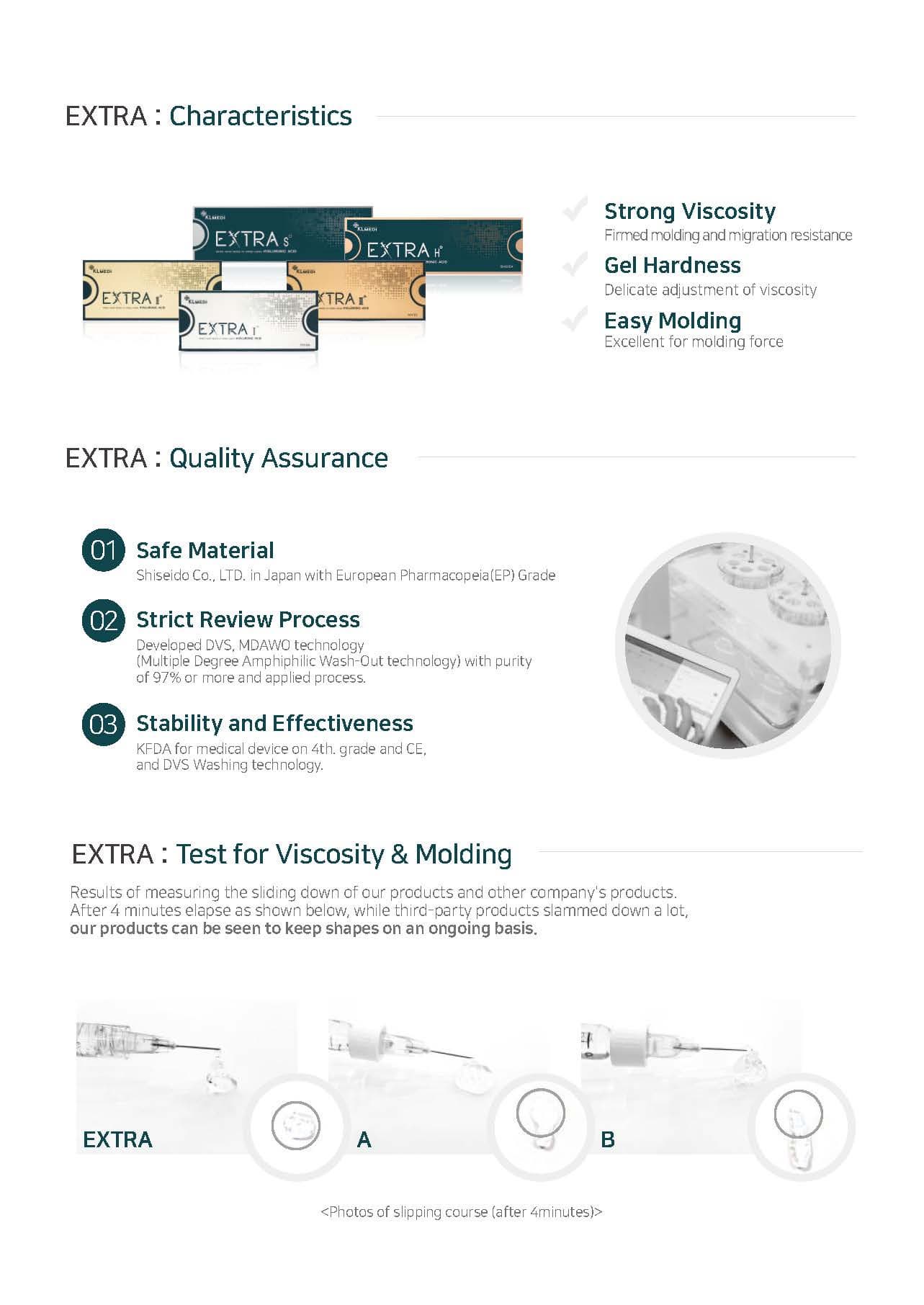
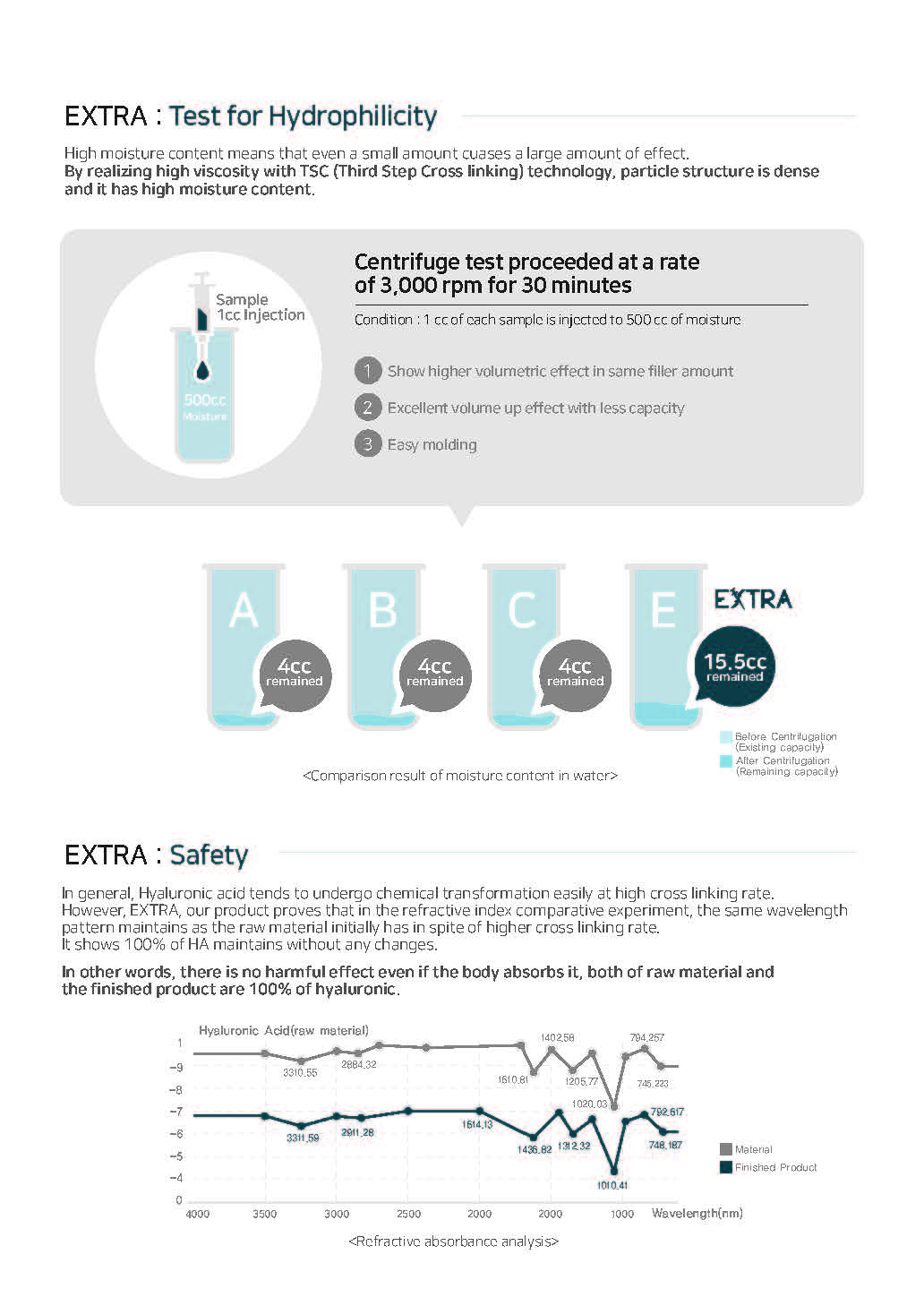
EXTRA : Quality Assurance
1. Safe Material
Shiseido Co., LTD. in Japan with European Pharmacopeia(EP) Grade
2. Strict Review Process
Developed DVS, MDAWO technology (Multiple Degree Amphiphilic Wash-Out technology) with purity of 97% or more and applied process.
3. Stability and Effectiveness
KFDA for medical device on 4TH grade and CE, and DVS Washing technology.

Innovative Technology
Third Step Cross linking(TSC)
Third Step Cross linking(TSC) maximizes viscosity through a patented 3step microbeads process.
The technology to increase cross-linking rate, it has increased the sustainability of HA.
We adjusted cross-linking rate according to product characteristics. We produced a various of high quality EXTRA depending on area.
DVS (Divinyl Sulfone)
cross-linked HA
EXTRA has developed MDAWO technology (Multiple Degree Amphiphilic Wash-Out technology) and applied it to processes.
The process of technology, the DVS component is completely removed and none of un-cross linked DVS is detected.
In general, the crosslinking process is carried out using DVS having a purity of about 95% for BDDE used for crosslinking HA and 97% or more purity for EXTRA. By using highly pure crosslinking bridge, crosslinking ratio is high, and high quality products can be produced.
EXTRA : Quality Assurance
1. Safe Material
Shiseido Co., LTD. in Japan with European Pharmacopeia(EP) Grade
2. Strict Review Process
Developed DVS, MDAWO technology (Multiple Degree Amphiphilic Wash-Out technology) with purity of 97% or more and applied process.
3. Stability and Effectiveness
KFDA for medical device on 4TH grade and CE, and DVS Washing technology.
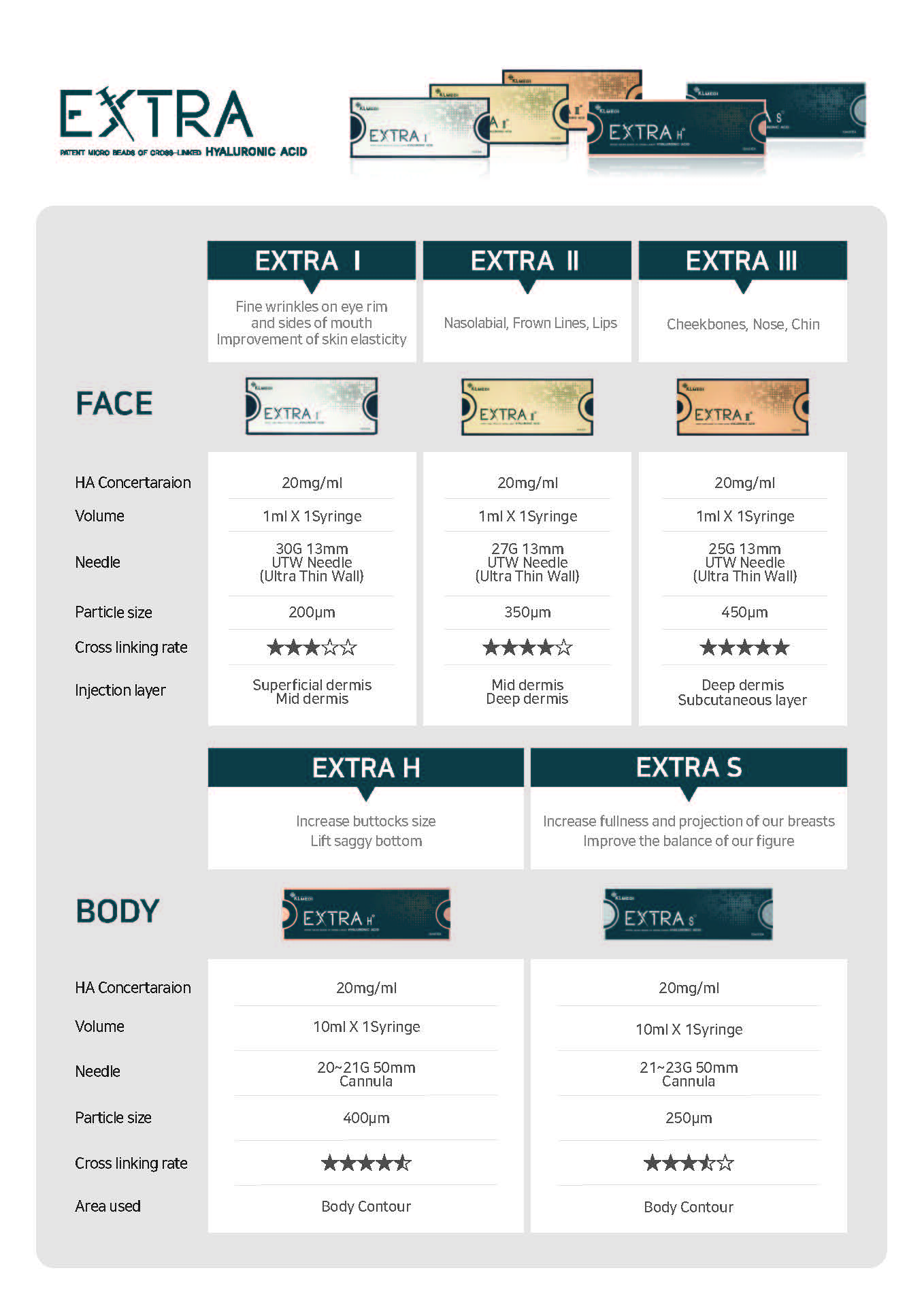
Face : Areas Used
EXTRA can be used for specific areas, ensures a safe and effective result.
EXTRA Ⅰ
Fine wrinkles on eye rim and sides of mouth
Improvement of skin elasticity
EXTRA Ⅱ
Nasolabial, Frown Lines, Lips
EXTRA Ⅲ
Cheekbones, Nose, Chin
Body : Areas used
EXTRA S
1. Increase fullness and projection of our breasts.
2. Improve the balance of our figure.
EXTRA H
1. Increase buttocks size.
2. Lift saggy bottom.
- Verified Certificate
-

B2B Trade
| Price (FOB) | Negotiable | transportation | - |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | T/T | Shipping time | Negotiable |
- President
- Changwoo, Lee
- Address
- Seongsui-ro 10-gil,703~705 Ace High-End Seongsu Tower 14, Seongdong-gu, Seoul, Korea
- Product Category
- Beauty & Personal Care,Beauty Equipment
- Year Established
- 2005
- Company introduction
-
Established in 2005, K.L Global has been supplying product to various sales channel andnetworks. We import, export and manufacture the leading trends skin cares and medical products with quality oriented.
We also made continuous efforts to developing and searching new and innovative items tointroduce to the market as leader which we aim for.
We also assist our clients based on trust, reliability, integrity and commitment for maintaininglong-term relationship.
- Main Product
Related Products
VTM, UTM, Viral Transport Media, EnTM Collection and Transport System

Wound Care bandages for diabetic

Disposable Bladeless Trocars [Enpole & Enpole pro] Slim Simple Laparoscopic trocar

StiMus Hydrogel Electrode

Fast-Aid Wound Care Cartoon bandage Children pack