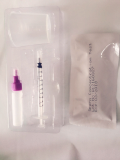
One Step Accurate/Medical/Home/Easy CEA Serum Rapid Test Kit
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- China
- Brand name
- MB
- Payment Terms
- MoneyGram,Others,T/T,Western Union
- Production method
- Negotiable
- Shipping / Lead Time
- Negotiable / Negotiable
- Keyword
- rapid test, cea, accurate rapid test, cea rapid test

Mex Biotech Hong Kong Ltd
- Verified Certificate
-
12

| Product name | One Step Accurate/Medical/Home/Easy CEA Serum Rapid Test Kit | Certification | - |
|---|---|---|---|
| Category |
Medical Devices
Medical Test Kit Other Monitoring & Diagnostic Equipment |
Ingredients | - |
| Keyword | rapid test , cea , accurate rapid test , cea rapid test | Unit Size | - |
| Brand name | MB | Unit Weigh | - |
| origin | China | Stock | - |
| Supply type | - | HS code | - |
Product Information
INTENDED USE
The CEA Gold Rapid Screen Test (RST) is a chromatographic immunoassay for the detection of cancer embryo antigen (CEA) in human serum or plasma.
PRINCIPLE
A correlative antigen to intestinal cancer, it can be used to assay the curative effect and prognosis of colon cancer.To some malignant cancer or pulmonary carcinoma outside of the intestine, the concentration of CEA in serum is ahigh. In clinical, CEA is taken as an early index to colon cancer, carcinoma of stomach, rectal cancer, cancer ofesophagus and relapse of cancer after the operation.
The CEA RST is a chromatographic immunoassay (CIA) for the detection of CEA in human serum or plasma.Specific antibody against CEA re-coated onto membrane as a capture reagent on the test region. During the test,specimen is allowed to react with the colloidal gold particles, which have been labeled with other specific antibodies.If CEA present, a pink colored band will develop on the membrane in proportion to the amount of CEA presented inthe specimen. Absence of this pink colored band in the test region suggests a negative result. To serve as aprocedural control, a pink colored band in the control region will always appear regardless the presence of CEA.This RST is convenient, easy to read, sensitive, rapid and credible diagnostic kit that suitable to all levels of hospitals and medical and hygienical institutions.
REAGENTS AND MATERIALS PROVIDED
1. One pouched cassette with desiccant.
2. One piece of operating instruction.
3. One bottle of whole blood chasing buffer.
WARNING AND PRECAUTIONS
1. For in vitro diagnostic use only.
2. Do not use after expiration date.
3. Test device should remain sealed until use.
4. The device stored at 4°C should be balanced to room temperature prior to use.
5. Desiccant in the sealed pouch, don’t eat it.
STORAGE
The kits should be stored at temperature 4-30°C, the sealed pouch for the duration of the shelf life (24 months).
Delivery Terms
1.We will ship the items after the payment is reached.
2.We can ship to you by UPS/DHL/TNT/Fedex. Pls contact us directly and we will use your preferred ways.
3.We are not responsible for any accidents, delaysor other issues that are the responsibility of the shipping service.
4.Any import fees or charges are the buyer's responsibility.
Package
1)Neutral bulk package;
2)Neutral box package;
3)OEM package.
If you have some other requirements ,pls contact with us freely.
One-Step Tumor Marker Rapid Test is an immunochromatography based one-step in vitro diagnostics test for the qualitative determination of AFP, PSA and CEA in human serum or plasma.
Our Strength
1)Advanced production line
2)High standard quality conform to international market
3)Competitive price (factory direct price)
4)Integrated customer service and technology support
B2B Trade
| Price (FOB) | Negotiable | transportation | - |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | MoneyGram,Others,T/T,Western Union | Shipping time | Negotiable |

- President
- Encore
- Address
- Room 1017 Tongxinyuan Liantang Luohu Shenzhen Guangdong
- Product Category
- Medical Devices,Medical Test Kit,Other Monitoring & Diagnostic Equipment
- Year Established
- 2001
- No. of Total Employees
- 51-100
- Company introduction
-
Mex Biotech Hong Kong Ltd.is a growing supplier for in-vitro diagnostics product.Develop,manufacture and distribute in-vitro rapid diagnostic test products.We have developed long-term business relationships with clients all over the world.Honesty is a basic principle of our company. Our company is committed to providing high quality products to customers, to customers as the center, do our best to meet customers'demands.Our aim is to satisfy our Long-term cooperation customers and welcome new clients.
We are currently providing an extensive range of immunochromatography based one-step rapid tests as below:
1)Fertility Hormone for HCG,LH ,FSH;
2)Infectious diseases & STD for HBsAg, Anti-HBsAb, HCV, HIV 1/2, HIV saliva,Syphilis, Malaria, dengue, H.pylori,TB,Chlamydia,Conorrhea,Typhoid,Rotavirus, Adenovirus;
3)Tumor markers for AFP, CEA, PSA, FOB;
4)Cardiac markers for Troponin I & Cardiac 3 in 1 (Troponin I, Myoglobin, CK-MB);
5)Urine reagent rapid test ,such as URS 1-12 parameters .
We appreciate for your interest in our products and looking forward more cooperation in near future.
- Main Markets
-
 Malaysia
Malaysia
 Myanmar
Myanmar
 Philippines
Philippines
 Saudi Arabia
Saudi Arabia
 Singapore
Singapore
 South Korea
South Korea
 Sri Lanka
Sri Lanka
 Turkey
Turkey
- Main Product



































 China
China