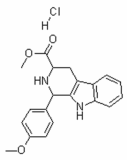
General Toxicology
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- U.S.A
- Brand name
- Creative Animodel
- Payment Terms
- Negotiable
- Production method
- Negotiable
- Shipping / Lead Time
- Negotiable / Negotiable
- Keyword
- toxicology services, general toxicology
- Category
- Pharmaceutical Intermediates
Creative Animodel
- Verified Certificate
-
11

| Product name | General Toxicology | Certification | - |
|---|---|---|---|
| Category | Pharmaceutical Intermediates | Ingredients | - |
| Keyword | toxicology services , general toxicology | Unit Size | - |
| Brand name | Creative Animodel | Unit Weigh | - |
| origin | U.S.A | Stock | - |
| Supply type | - | HS code | - |
Product Information
Overview
In the area of general toxicology, Creative Animodel offers our customers standard protocols designed to support approval submissions to domestic and international regulatory agencies. With multiple test species available, studies are overseen by Study Directors with vast experience gained from performing over 1000 GLP studies. From standard safety studies to more specialized services, Creative Animodel can help you achieve your study goals more quickly and with greater confidence.
General Toxicology Services
Creative Animodel provides a comprehensive offering of general toxicology services at our facilities, including:
Acute Toxicity
Creative Animodel performs acute toxicity tests which describe the adverse effects of a substance that result either from a single exposure or from multiple exposures in a short space of time (usually less than 24 hours).
Accumulation Toxicity
The accumulation and their metabolic byproducts in organs can be toxic, leading to organ damage. Creative Animodel conducts standard toxicology protocols to test accumulation toxicity.
Subchronic Toxicity
Studies that continue for 90 days or for up to 10% of a test subject's life span are considered subchronic. Creative Animodel provides expertise in the planning, design, conduct, reporting and filing of subchronic toxicity tests.
Chronic Toxicity
Chronic toxicity is a property of a substance that has toxic effects on a living organism, when that organism is exposed to the substance continuously or repeatedly. Our AAALAC-accredited facilities are equipped with the most advanced technologies to provide our clients with chronic toxicity tests to make better development decisions.
Administration Routes:
| ---Oral Gavage Dietary Capsule Drinking water | ---Other Dermal Intra-colonic Intradermal Intravesicular Intravitreal Ocular Sublingual ---Continuous Infusion |
| ---Parenteral Intramuscular Intraperitoneal Intravenous Subcutaneous |
Our work is GLP compliant and AAALAC-accredited. Plus, we have a thorough understanding of global regulatory issues and can help ensure that you are conducting the right studies at each step of the process. Moreover, our facilities are outfitted with the very latest technology for data measurement, data collection and animal care. Please let us know how we can help you.
B2B Trade
| Price (FOB) | Negotiable | transportation | - |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | Negotiable | Shipping time | Negotiable |
- President
- Mr.Zhang
- Year Established
- 2005
- Company introduction
-
Creative Animodel totally understands that it is a challenging and long-time process to develop novel therapies especially anticancer regimens. As an experienced bioscience CRO, we would like to help you accelerate the process through our preclinical services including PD, PK, toxicology services and the establishment of tumor-bearing animal models, the supply of tumor cell lines and tissues, and the support of tumor related biotechniques.
Our highly experienced preclinical team has performed a huge number of PD, PK, and toxicology experiments to evaluate novel compounds. According to our detailed protocol, specific aspects of drug property and its mechanism of action are fully understood. During researches, the study results will be sent to clients in a timely and cost-effective manner. If necessary, clients can also work with our PD, PK or toxicology team to design the most appropriate protocol and monitor the whole experimental process.
In the past years, Creative Animodel has collected more than 80 cases of human tumors. At present, 30 cases are growing as subcutaneous xenografts in nude mice, and others were retained as nitrogen frozen samples. Tumors types comprise: stomach, lung, liver, pancreas, kidney, bladder, skin, breast, brain, colon, prostate, sarcomas (bone), uterus, ovary and hematological models. Also, we have a full range of human tumor cell lines that have been validated in our in vivo efficacy studies or reported in literature. According to the requirements from clients and our experience, Creative Animodel can offer subcutaneous, orthotopic, metastatic, transgenic and chemoinduced models using these tumor samples and cell lines.
Additionally, Creative Animodel provides powerful tumor-related biotechniques support for clients. Our services focus on in vitro and in vivo preclinical studies as well as toxicological, pathological, and toxicokinetics researches.
Creative Animodel has constructed systematic data and experience in pharmacology or oncology services. If you are interested in our laboratory, please contact us. Our research scientists will work closely with you to develop detailed study protocols to meet your goals.
- Main Markets
-
 Australia
Australia
 Canada
Canada
 Singapore
Singapore
 South Korea
South Korea
 U.S.A
U.S.A
- Main Product