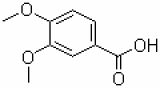
Apixaban intermediates / CAS 27143-07-3
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- China
- Payment Terms
- Negotiable
- Production method
- Negotiable
- Shipping / Lead Time
- Negotiable / Negotiable
- Keyword
- 1575-61-7, 27143-07-3, 503615-07-4, 545445-44-1
- Category
- Pharmaceutical Intermediates

Nanjing CHICO pharmaceutical Co.,Ltd.
- Verified Certificate
-
11

| Product name | Apixaban intermediates / CAS 27143-07-3 | Certification | - |
|---|---|---|---|
| Category | Pharmaceutical Intermediates | Ingredients | - |
| Keyword | 1575-61-7 , 27143-07-3 , 503615-07-4 , 545445-44-1 | Unit Size | - |
| Brand name | - | Unit Weigh | - |
| origin | China | Stock | - |
| Supply type | - | HS code | - |
Product Information
Product Name:Ethyl
chloro[(4-methoxyphenyl)hydrazono]acetate
CAS No.:2
Appearance: Pale yellow solid
Assay:≥98%
Apixaban Intermediates
|
CAS No.: |
Product Name |
|
1575-61-7 |
5-Chlorovaleryl chloride |
|
2 |
Ethyl chloro[(4-methoxyphenyl)hydrazono]acetate |
|
536759-91-8 |
4,5,6,7-Tetrahydro-1-(4-methoxyphenyl)-6-(4-nitrophenyl)-7-oxo-1H-pyrazolo[3,4-c]pyridine-3-carboxylic
acid ethyl ester |
|
50 |
ethyl 6-(4-aminophenyl)-1-(4-methoxyphenyl)-7-oxo-4,5-dihydropyrazolo[3,4-c]pyridine-3-carboxylate |
|
545445-44-1 |
5,6-Dihydro-3-(4-morpholinyl)-1-[4-(2-oxo-1-piperidinyl)phenyl]-2(1H)-pyridinone |
|
503612-47-3 |
Apixaban |
B2B Trade
| Price (FOB) | Negotiable | transportation | - |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | Negotiable | Shipping time | Negotiable |

- President
- David Wu
- Address
- No.9 Weidi Road-Jiangsu life science and technology Innovation Park,Nanjing,China
- Product Category
- Organic Intermediate,Other Pharmaceutical Chemicals
- Year Established
- 2008
- No. of Total Employees
- 1-50
- Company introduction
-
Established in 2008, Nanjing CHICO pharmaceutical Co.,Ltd. serving global pharmaceutical industry with best quality products and professional services. We offer APIs and intermediates with effective technical support and professional documentation service.
We have our teams that leaded by lots of pharmaceutical and chemical experts and professors which closely following the international new drug research and development dynamic that help us to provide a strong continuous innovation, and make us always stand in the forefront of new drug research and development.
Gradually we form a targeted anti-tumor, anticoagulant, anti-diabetes medicine raw materials and intermediate products system.
We have complete production chain of gram-kilogram-ton so that we can achieve rapid response in the whole R&D-scale up- producing process for new product and transform the technology into productivity at the first time.
We always regard the quality as life, so we establish a sound quality assurance system and strict quality control standards according to GMP requirements.We are adhering to the "Consummate service Convincing honor" business strategy and providing best quality products, competitive price ,professional service, and strict working style to meet our customer's need, reduce its product cost by strong technical accumulation and outstanding professionalism to face our customer's market challenges. We take our customers' success as our own success.
- Main Markets
-
 Germany
Germany
 India
India
 Iran
Iran
 Italy
Italy
 South Korea
South Korea
- Main Product



































 China
China