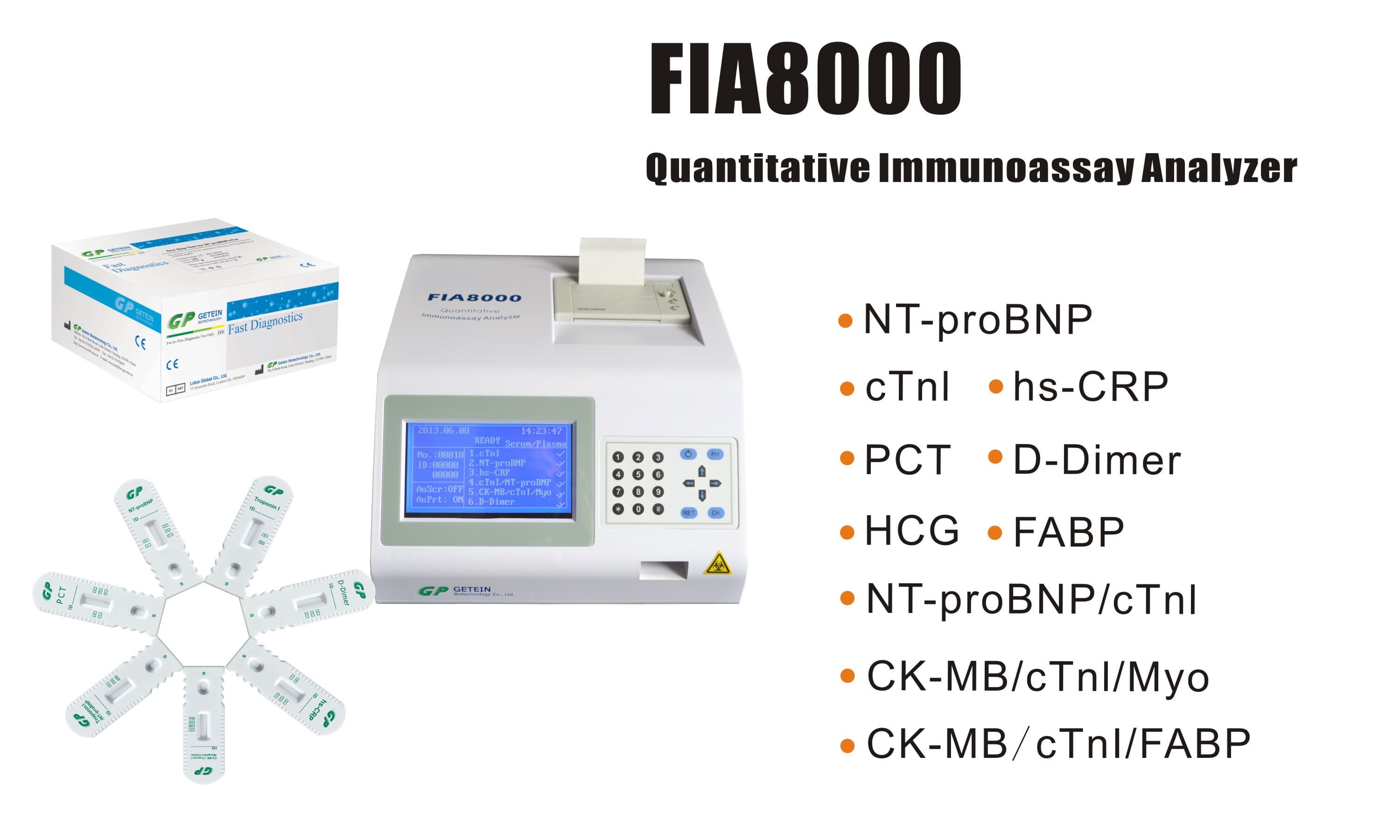
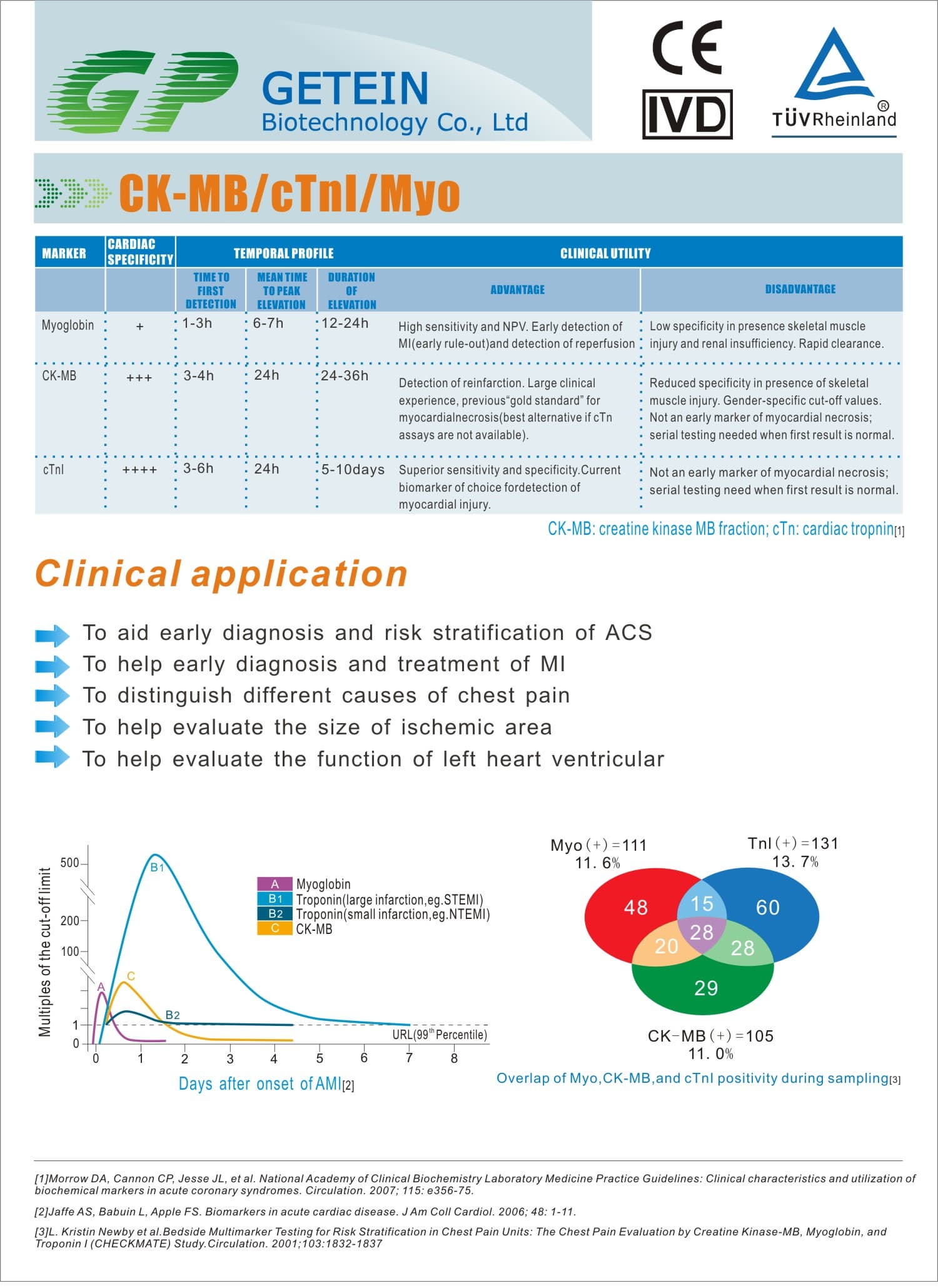
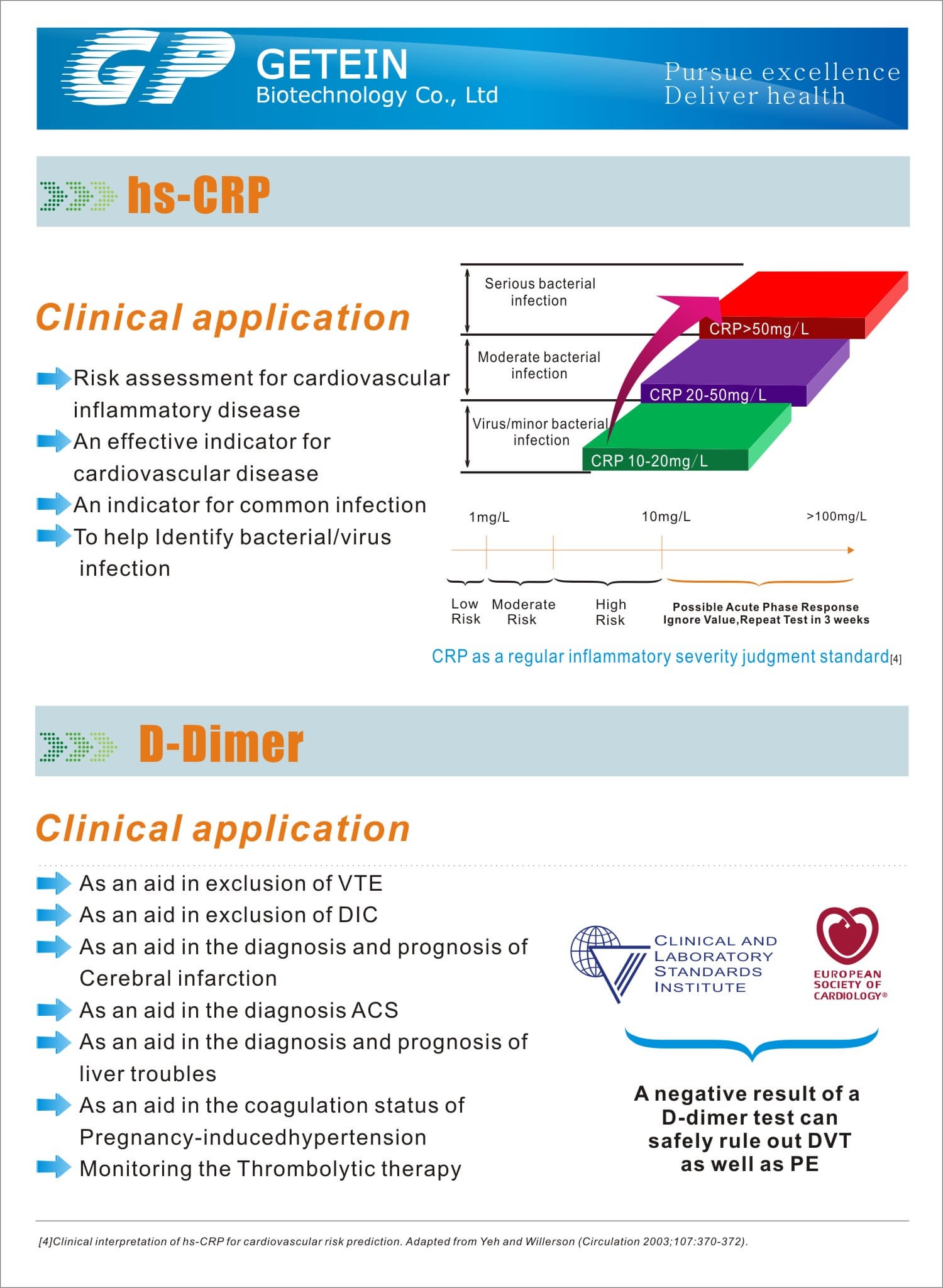
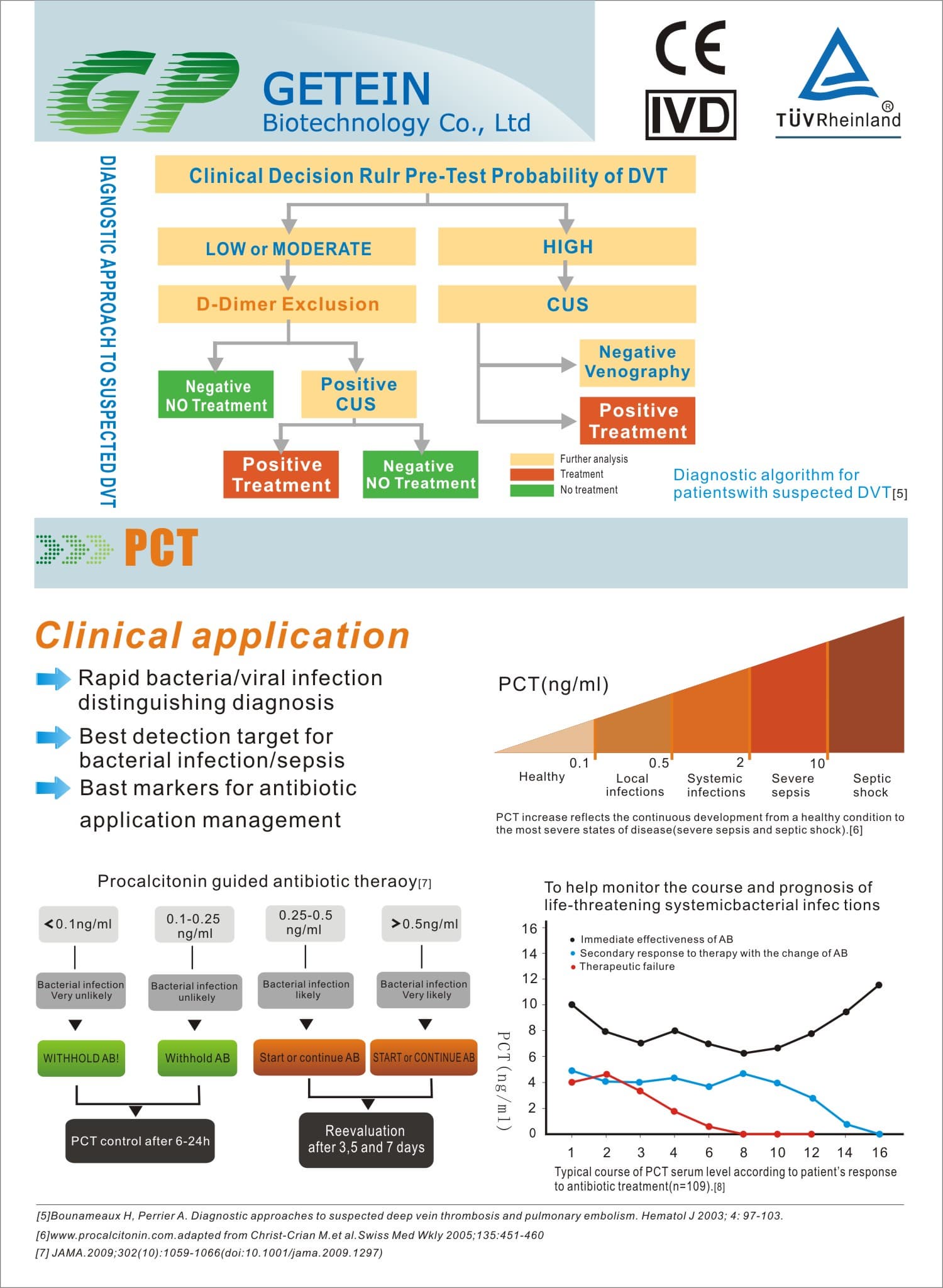
FIA8000 Quantitative Immunoassay Analyzer
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- China
- Payment Terms
- Negotiable
- Production method
- Negotiable
- Shipping / Lead Time
- Negotiable / Negotiable
- Keyword
- ctni ck-mb myoglobin, nt-probnp, pct, d-dimer
- Category
- Medical Analyzer , Multi-Parameter Monitor

Nanjing Getein Biotechnology Co.,Ltd
- Country / Year Established
-
 China
/
2002
China
/
2002
- Business type
- Manufacturer
- Verified Certificate
-
10

| Product name | FIA8000 Quantitative Immunoassay Analyzer | Certification | - |
|---|---|---|---|
| Category |
Medical Analyzer
Multi-Parameter Monitor |
Ingredients | - |
| Keyword | ctni ck-mb myoglobin , nt-probnp , pct , d-dimer | Unit Size | - |
| Brand name | - | Unit Weigh | - |
| origin | China | Stock | - |
| Supply type | - | HS code | - |
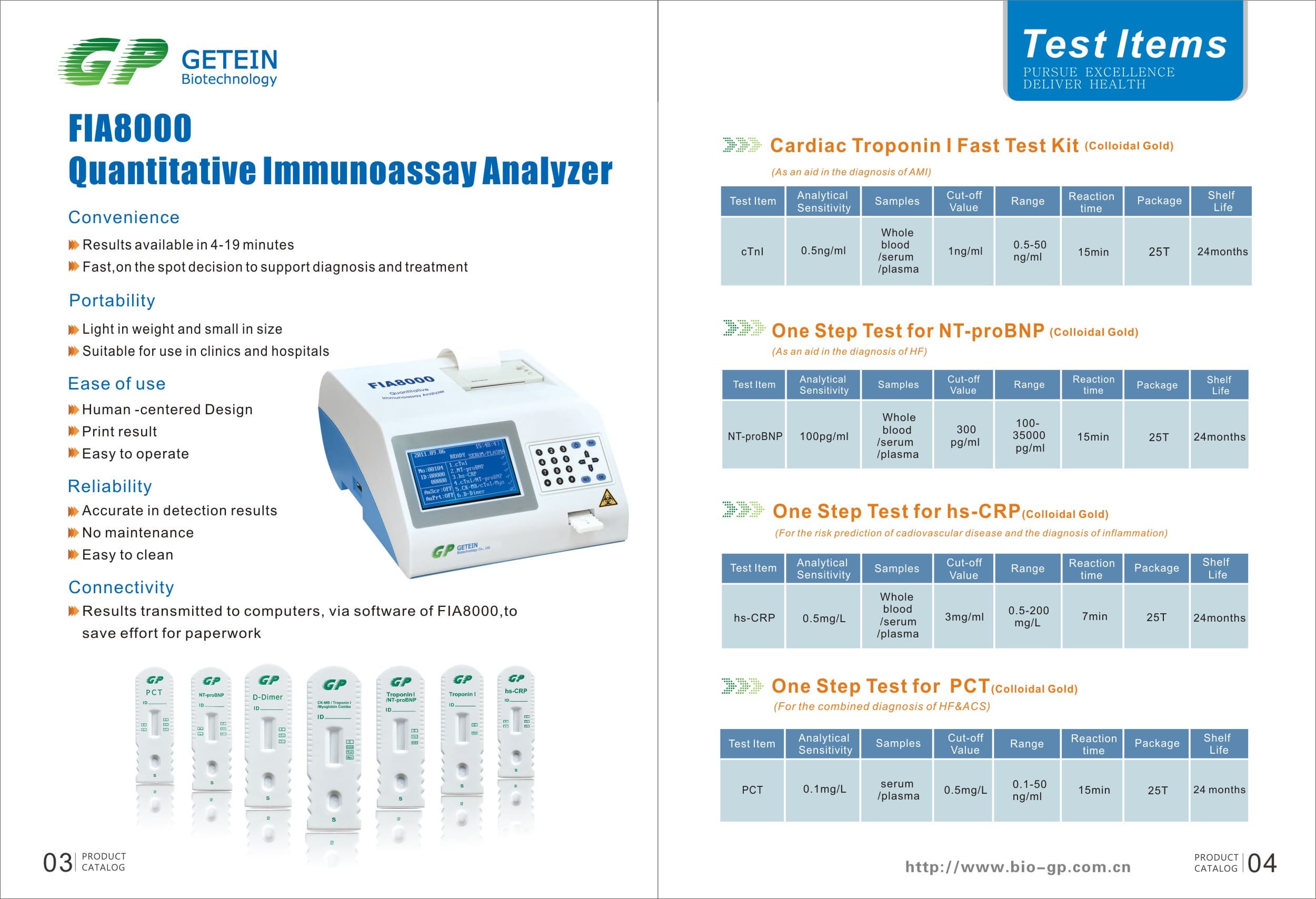
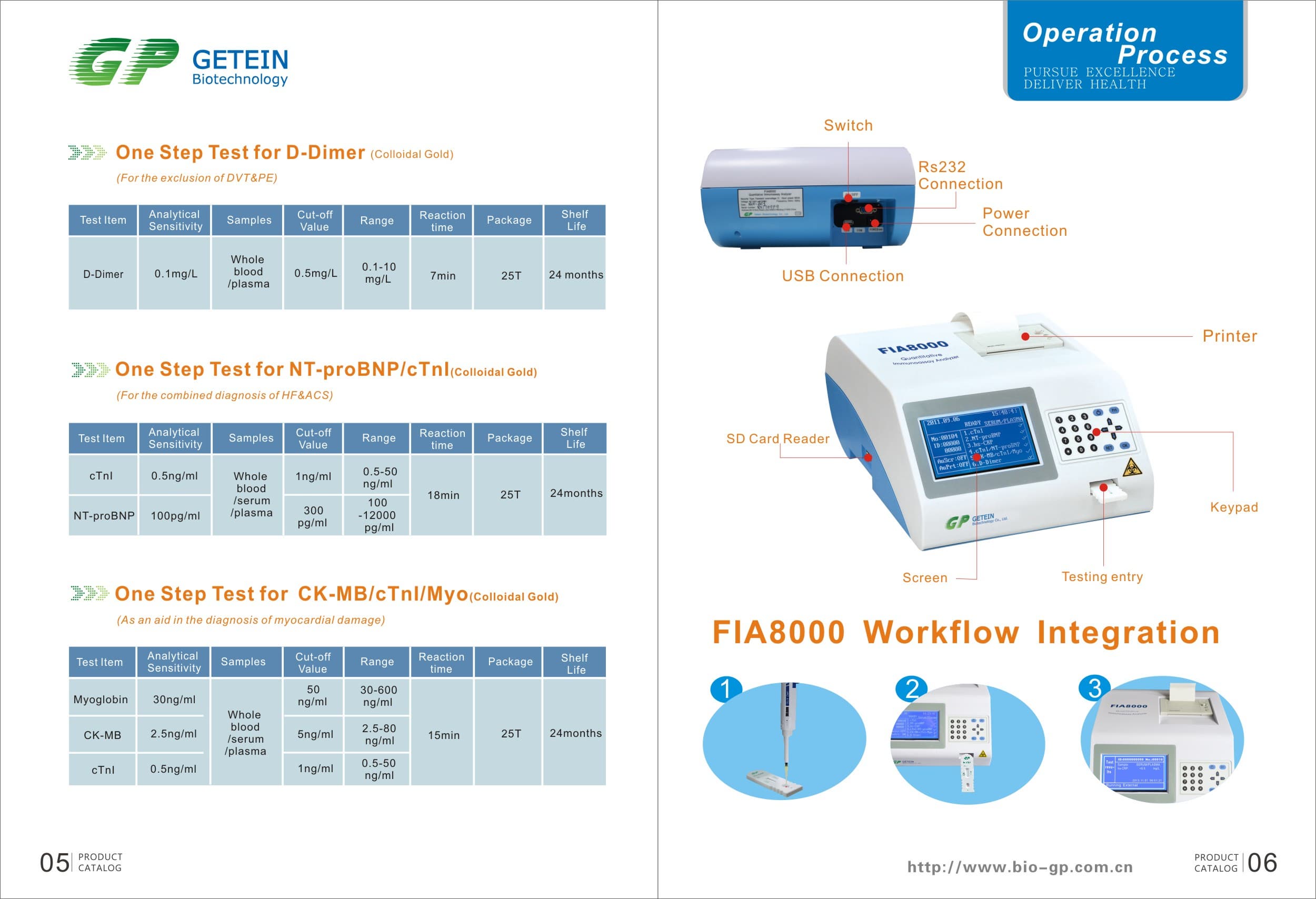
Product Information
Specs
Methodology | Colloidal gold | |
Test Item | cTnI, NT-proBNP, NT-proBNP/cTnI, CK-MB/cTnI/Myo, hs-CRP, PCT, D-Dimer | |
Sample | Serum/Plasma/Whole Blood | |
Background Count | The voltage must be higher than 3500mV when test a blank quality control card | |
Linearity | r≥0.99(0--4000mV) | |
Repeatability | CV≤1% | |
Stability | The voltage variation should be less than ±2% if test the same quality control card within in 1h. | |
Working Environment | Temperature | (+15°C~+35°C) |
Humidity | 10%~85% | |
Atmospheric Pressure | 70.0kPa~106.0kPa | |
Power Supply | AC100V~240V 50Hz~60Hz | |
Transport and Storage Environment | Temperature | (-10°C~+40°C) |
Humidity | ≤93% | |
Atmospheric Pressure | 50.0kPa~106.0kPa | |
B2B Trade
| Price (FOB) | Negotiable | transportation | - |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | Negotiable | Shipping time | Negotiable |

Nanjing Getein Biotechnology Co.,Ltd
- Country / Year Established
-
 China
/
2002
China
/
2002
- Business type
- Manufacturer
-
10

- President
- Su Enben
- Product Category
- Medical Analyzer,Multi-Parameter Monitor,Other Monitoring & Diagnostic Equipment
- Year Established
- 2002
- No. of Total Employees
- 101-500
- Company introduction
-
Getein Biotechnology Co., Ltd. was founded in March 2002, specializing in in-vitro diagnostic reagents and medical device. It is a modern high-tech company with R & D, production and sales teams all-in-one. We locate in Nanjing, a famous beautiful city as the capital of six dynasties, where is quite attractive to many talents. We now have about 600 employees, 4/5 are college degree or above, in which including more than 50 doctors and masters, as well as hundreds of R&D professionals.
Getein Biotech has GMP standard workshop of more than 2,000 square meters, advanced research and production equipment, and a 60 acres animal breeding base. All these above guarantee the safety and quality of raw materials and manufacturing process. In such case, we achieved the Rhine TüV ISO13485 quality management system certification and CE certification sequentially, which confirmed our capability of scientific and rigid production and quality management system to ensure quality, reliability and stability.
- Main Product
Related Products

Berry reusable nellcor oximax DB 9 Pins spo2 sensor
Multi Parameter Patient Monitor_IP4050

Patient Monitor VP-1000

Urine Analysis Test Strip Self-Stik Series
Vacuum Blood Collection Tube