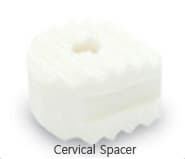
Cervical Spacer
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- Payment Terms
- Negotiable
- Production method
- Negotiable
- Shipping / Lead Time
- Negotiable / Negotiable
- Keyword
- allograft, medical device, medical supplies, xenograft
- Category
- Health & Medical , Medical Devices
| Product name | Cervical Spacer | Certification | - |
|---|---|---|---|
| Category |
Health & Medical
Medical Devices |
Ingredients | - |
| Keyword | allograft , medical device , medical supplies , xenograft | Unit Size | - |
| Brand name | - | Unit Weigh | - |
| origin | Stock | - | |
| Supply type | - | HS code | - |
Product Information
Cervical Spacer
│Description│
- Made of 100% human bone which removes the risk of allergic reaction or side-effects caused by artificial material.
- Anatomical/biocompatible design for effective bone fusion.
- Maintain the balance of force between upper and lower spine.
- Assured safety with Clearant Process® which guarantees sterilization and effectiveness for bone fusion.
- Reduced pain compared to Titanium cage.
- Ready with many sizes for wide range of patients
- Easy-to-use instrument
*Company Overview
01. Korea's Largest Human Tissue Bank
Since the establishment of Cellumed Human Tissue Bank in 1997, Cellumed has become the biggest tissue bank in the Republic of Korea. Cellumed also passed KFDA annual inspections as well as FDA site inspection for cGMP srandard.
Cellumed in especially well-known for Clean Room and Bio-Clearant technology which guarantees safe and effective tissue processing. The review of FDA inspectors showed that Cellumed's water system of Clean Room is' a world-class system'
02. Powered by consistent focus on R&D
As our society is aging along with the increase in various leisure activities, demand for ideal medical devices and orthopedic medical equipment is noticeably growing. Facing this increase in demand, Cellumed expanded into the global market by exporting EOS pedicle screw and continuing to develop B-P Kness artificial knee joint system followed by the acquisition of ENDOTEC and their advanced technology.
03. Powered by consistent focus on R&D
Cellumed has continuously made huge investment in R&D sector of the company to stimulate innovational technological development as well as to motivate researchers to develop perfect treatment for musculoskeletal diseases.
Cellumed has discovered and developed Bone Morphogenetic Protein (BMP) as an innovative way of enhancing bone regeneration through years of dedication in R&D. So far, one of the BMP2 related products has become commercialized.
Also, Rafugen BMP2 passed nonclinical trial and is currently in the process of clinical trial to be authorized and licensed for market entry. Cellumed's advanced technology became possible through out commitment in R&D and will surely become a growth engine for future business.
B2B Trade
| Price (FOB) | Negotiable | transportation | - |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | Negotiable | Shipping time | Negotiable |

Exhibition 1
preventive products-
11


- President
- You In Soo
- Address
- Chung-Dam-dong, Hakdong-Ro, 401, Geumha Building, 9th Floor, Gangnam-gu, Seoul, Korea
- Product Category
- Medical Devices
- Year Established
- 1997
- No. of Total Employees
- 51-100
- Company introduction
-
Cellumed Co., Ltd. has been steadily growing up and have become a frontier in the health care industry in 1997. We have been putting strenuous endeavor for the development of “better, safe, effective” medical device in order to make sound contribution to human health. Cellumed was one of the first bone banks established in Korea, and have been supplying human/bovine bone parts for various indications to our customers both domestic and overseas. L-F Fixed/Mobile Knee (TKR) made of titanium is being sold in the Korean market and now currently under the process of acquiring CE certificate. Our continuous investment on R&D has led us to address the FDA 510(k) registration of Rafugen DBM gel along with Bio BMP2 registered in Korea and South East Asian countries. We are looking for our very pertinent distributor whom Cellumed will share synergies with.
- Main Markets
-
 China
China
 Colombia
Colombia
 Dominican Rep.
Dominican Rep.
 India
India
 South Korea
South Korea
 U.S.A
U.S.A
- Main Product