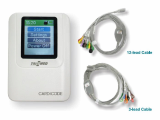
Ambulatory Nibp/SPO2/VITAPIA5500
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- Payment Terms
- Negotiable
- Production method
- Negotiable
- Shipping / Lead Time
- Negotiable / Negotiable
- Keyword
- Category
- ECG Machine

Trismed Co., Ltd.
- Verified Certificate
-
17



| Product name | Ambulatory Nibp/SPO2/VITAPIA5500 | Certification | - |
|---|---|---|---|
| Category | ECG Machine | Ingredients | - |
| Keyword | - | Unit Size | - |
| Brand name | - | Unit Weigh | - |
| origin | Stock | - | |
| Supply type | - | HS code | - |
Product Information
AMBULATORY NIBP/SPO/VITAPIA5500
- Transfer data to PC-baded Software via USB port, accurate analysis systems.
- Compact and portable design
- Adjustable audible and visual alarms
- 2.4" TFT display of NIBP, SPO2 and pulse rate
- Real-time ambulatory monitoring
- 24hours storage and review of data
- PC networking on the windows based S/W for review and print
- Up to 300 items of memroy for common user and 358 for ambulatory blood pressure monitoring user.
- Clinically proven accuary Battery status indicator
- Automatic cuff inflation and deflation
- Reliable power supply with 2AA batteries
B2B Trade
| Price (FOB) | Negotiable | transportation | - |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | Negotiable | Shipping time | Negotiable |

- President
- Hoon-Kyu Lee
- Address
- 409 SMECA, Yuseong-gu, Daejeon, Korea
- Product Category
- Other Examination & Testing Instrumnet
- Year Established
- 2014
- No. of Total Employees
- 1-50
- Company introduction
-
With company's philosophy of customer Satisfaction, Superiority and Specialization, TRISMED CO., LTD. was founded in 2000 and has been active in the research and development, manufacture and distribution on a world wide scale of cardiac diagnosis systems.
The wide-range products are targeted to offer reasonable price, international standard performance and high quality with our strong competence and deep experience.
TRISMED believes in coming true the leader in the cardiac diagnosis systems such as various Electrocardiographs(ECG), patient monitors and pulse oximeter in the world-wide.
Our quality system complies with the international quality standard - EN ISO13485:2003 and EC Council Directives 93/42/EEC CE(MDD).
- Main Markets
-
 Austria
Austria
 Romania
Romania
 Spain
Spain
 Turkey
Turkey
 U.S.A
U.S.A
- Main Product
Related Products
D700 Monitor Defibrillator
PACSPLUS MammoQC
TAB electrodes / Resting ECG electrodes

ECG-8112 ECG Machine

Digital Physiograph P400



































 South Korea
South Korea