Tennis / Golfer’s Elbow Wrap With Pressure Pad DR-E019
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- South Korea
- Payment Terms
- Negotiable
- Production method
- Negotiable
- Shipping / Lead Time
- Negotiable / Negotiable
- Keyword
- tennis elbow support, drmed, golf elbow, dr-e019
- Category
- Medical Consumables
| Product name | Tennis / Golfer’s Elbow Wrap With Pressure Pad DR-E019 | Certification | - |
|---|---|---|---|
| Category | Medical Consumables | Ingredients | - |
| Keyword | tennis elbow support , drmed , golf elbow , dr-e019 | Unit Size | - |
| Brand name | - | Unit Weigh | - |
| origin | South Korea | Stock | - |
| Supply type | - | HS code | - |
Product Information
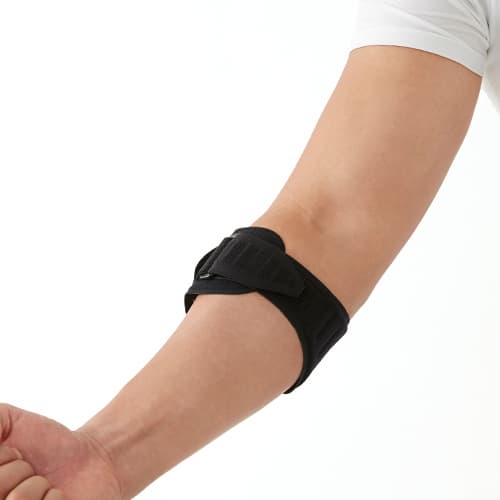
Dr.MED Tennis&Golfer’s Elbow Wrap With Pressure Pad
4-step classes by supportstrength of A-MI Global.,Co.Ltd.
Dr.MED products are classified as four classes.
The various products can cover preventing injuries to rehabilitation after surgery.
CLASS 2_MIDDLE SUPPORT
Indication
- Tennis elbow (Lateral epicondylitis)
- Golfer’s elbow (Medial epitrochleitis)
Features
- Allowing excellent support & compression on the elbow region.
- Adjustable compression by side Velcro Strap
- Easy & simple wear by wrap buckle style.
- Detachable compression pad reduces the pain caused by tennis/Golfer’s elbow
Fibers&Materials
PU, EVA, POM, Polyeseter, Nylon, Rubber
Size

B2B Trade
| Price (FOB) | Negotiable | transportation | - |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | Negotiable | Shipping time | Negotiable |

- President
- YU SIL GEUN
- Address
- Jangrimbeonyeong-ro 104th street,22, Saha-gu, Busan, Korea
- Product Category
- Other Health Care Products,Rehabilitation Therapy products
- Year Established
- 1965
- No. of Total Employees
- 51-100
- Company introduction
-
We, AMI GLOBAL.CO.,LTD, have been a global medical device company specialized and experienced in designing, manufacturing & distributing in the field of comfortable orthopedics, rehabilitation, healthcare and sporting supports since June, 1965 with its medical line, Dr.MED and sports line, SPOMED.
The operating system of factories is accredited by ISO9001/ISO14001/ISO13485 and its products are certified by FDA(USA) in 2003, CE mark in 2006 and GMO(medical device).
Our own brand Dr.MED has become various international partners for all those working at medical support/brace all over the world and will bring you improvement of person's life quality, comfort and pleasure.
- Main Markets
-
 Australia
Australia
 Bahrain
Bahrain
 India
India
 Japan
Japan
 Jordan
Jordan
 Kuwait
Kuwait
 Laos
Laos
 Lebanon
Lebanon
 Malaysia
Malaysia
 Mongolia
Mongolia
 Myanmar
Myanmar
 Qatar
Qatar
 Romania
Romania
 Russia
Russia
 Saudi Arabia
Saudi Arabia
 Singapore
Singapore
 U. Kingdom
U. Kingdom
 U.A.E.
U.A.E.
 U.S.A
U.S.A
 Viet Nam
Viet Nam
- Main Product
Related Products
_2.jpg)
SUMFREE HEALTH FACE MASK (KF94, KF80)
_2.png)
Biodegradable Vinyle(PLA)

Surgical / Medical Gown

M.Smart needle cannula grinding machine line
_2.png)
Biodegradable straws(PLA)





































 South Korea
South Korea


_DR-W136_2.jpg)


